Injured Motor Neurons
Contents
- A. Injured Motor Neurons: Changes in RNA, DNA and proteins
- 1. Do the axotomy-induced changes in specific proteins differ in the injured ventral root and spinal cord?
- 2. How do these proteins change within axotomized spinal motor neuron cell bodies?
- 3. How much does the total protein content of spinal motor neuron cell bodies increase after ventral root transection?
- 4. Is the increase in protein content a consequence of increased protein synthesis?
- 5. How much is total RNA synthesis altered after ventral root transection?
- 6. Does DNA synthesis occur in injured motor neuron cell bodies?
- B. Motor Neuron Size After Injury: Morphological and Biochemical Relationships
- 1. What is the magnitude and time course of changes in nucleolar, nuclear and cell body size after axotomy?
- 2. Do large motor neurons undergo the same relative increases in nucleolar, nuclear and cell body size as small motor neurons?
- 3. Are changes in the cytoskeleton after axotomy responsible for the conjugate enlargement of the nucleolus, nucleus and cell body?
- 4. Is protein added to the cell body cytoskeleton after axotomy?
- 5. Does an increase in total transcription in spinal motor neurons always lead to increased nucleolar, nuclear and cell body size and protein content?
- 6. Do changes in the cytoskeleton underlie increased nuclear eccentricity after axotomy?
- 7. Does damming up of axonal cytoskeleton cause increased nuclear eccentricity, chromatolysis and cell body enlargement after axotomy?
- 8. What signals the cell body that its axon has been injured?
- 9. Do motor neurons on the opposite side of the spinal cord from a unilateral ventral root injury respond to axotomy?
Early in our laboratory’s work, we sought ways to alter the morphology and biochemistry of spinal motor neuron cell bodies in experimental animals and to measure those changes quantitatively. The motor neuron’s response to axonal injury – the “axon reaction” – was an obvious choice, since the spinal motor axon is accessible outside of the central nervous system. When the axon of a mature motor neuron is damaged, the affected cell body undergoes many transient structural and biochemical changes. Unless the injury site is close to the cell body, spinal motor neurons usually survive the injury and regrow their axon. Our laboratory focused much of its attention on the characterization and relationships of the morphological and biochemical responses of motor neurons to axonal injury. We reasoned that these studies could be useful in several ways:
- The research could shed light on the mechanisms underlying peripheral nerve regeneration, which are still not well understood;
- Finding expected morphological and biochemical changes in motor neuron cell bodies isolated from the side of a unilateral ventral root injury would demonstrate that the cell bodies we isolate had projected their axons through the ventral root, thereby strengthening their identification as motor neurons;
- Since axotomy causes cell body enlargement, the work could help us understand the factors that control and sustain the large size of spinal motor neuron size, issues that are pertinent to normal and diseased motor neurons;
- Finally, a clearer understanding of the axon reaction could aid research on motor neuron disease. Axonal transport is slowed in models of ALS (Williamson and Cleveland, 1999) and could cause motor neuron death, especially if axonal transport is disrupted close to the diseased cell body (Lieberman, 1971). Moreover, morphological features of the axon reaction, such as central chromatolysis and dendritic atrophy can appear in amyotrophic lateral sclerosis (Nakano and Hirano, 1987), especially in rapidly progressing cases (Hirano and Iwata, 1979).
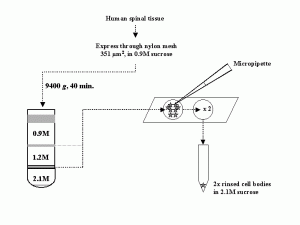
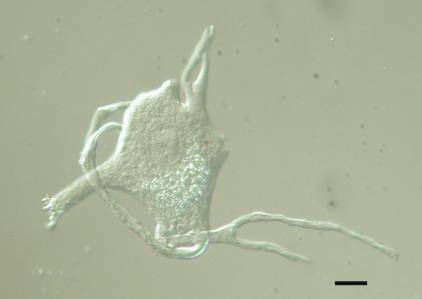
Unlike peripheral nerve injuries, damage to the ventral root spares most sensory fibers, which usually reach the spinal cord through the dorsal root. All of our studies on axotomized motor neurons used the grass frog (R. pipiens and its close relatives). The frog’s largely cartilaginous spinal column makes surgical access to the ventral roots via a dorsal laminectomy relatively easy and their lumbar ventral roots contain motor axons thought to be exclusively from alpha motor neurons (Gray, 1957). An experienced individual can perform six dorsal laminectomies in an afternoon.
A. Injured Motor Neurons: Changes in RNA, DNA and proteins
Before examining isolated motor neuron cell bodies, we quantified changes in four proteins in injured ventral roots and spinal cord, as a general survey of the magnitude and time course of their changes in the axotomized frog.
1. Do the axotomy-induced changes in specific proteins differ in the injured ventral root and spinal cord?
In a single series of experiments, we looked for axotomy-induced, time-dependent changes in four specific proteins – acetylcholinesterase, choline acetyltransferase, 6-phosphoguconate dehydrogenase, and tubulin – in the frog spinal cord and in the ventral root proximal to the site of axotomy on the injured side of the animal. Each of these four proteins was known to be affected by motor axonal injury. In addition, we quantified the total protein/wet weight of spinal cord and proximal ventral roots in the same tissue samples. Following transection of the left 9th and 10th ventral roots at the site of their exit from the spinal column, tissue samples were collected at postoperative days 2, 4, 6, 10, 20, 35, 55 and 75 and compared to samples from unoperated and sham-operated (0 and 20 days) frogs. Reinnervation of skeletal muscle by the injured motor axons has been reported to begin by postoperative day 50- 55 (Farel, 1978). The time course studies on choline acetyltransferase and 6-phosphoguconate dehydrogenase have not been published.
Acetylcholinesterase (AChE). We found significant increases (about 80% higher than contralateral and sham-operated controls) in this enzyme activity proximal to the injury site in the ventral roots 8 hours following axotomy, as the fast-transported enzyme appeared to dam up behind the sealed end of the cut axon (Sinicropi et al., 1982). This was the earliest detected change in any of the four proteins quantified in this study. The buildup of AChE activity continued for three days, reaching 300% of control values, and then abated, with normal levels recorded by postoperative day 10 and decreased levels as low as 60% of normal between days 20 and 55. In lumbar spinal cord ipsilateral to the site of injury, no significant change in AChE activity was detected until postoperative day 10, when AChE was decreased and remained so until about day 75.
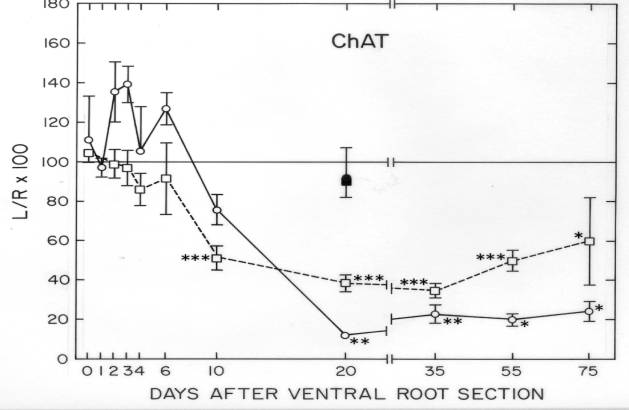
Choline acetyltransferase (ChAc). The activity of this enzyme, which synthesizes the motor neuron’s transmitter, ACh, was measured radiometrically (Sinicropi et al., 1989). During the first six days following ventral root transection, ChAc activity was elevated in the proximal ventral root by as much as 40% over contralateral and sham-operated controls, but statistical significance was not reached during this early period in our experiments ![]() . On the other hand, a significant decline to about 20% of normal ChAc activity was detected proximal to the site of injury by postoperative day 20 and was sustained to at least postoperative day 75. Similarly, ChAc activity declined sharply in the ipsilateral lumbar spinal cord by day 10, reaching a low of about 40% of its normal value by day 20, and recovered to about 60% of its normal level by postoperative day 75. ChAc was the only protein among the four analyzed in this study in which changes in the ipsilateral lumbar spinal cord served as a reasonably good indicator of changes in ventral root activity. With the other three proteins, most of the detectable change occurred in the ventral root only.
. On the other hand, a significant decline to about 20% of normal ChAc activity was detected proximal to the site of injury by postoperative day 20 and was sustained to at least postoperative day 75. Similarly, ChAc activity declined sharply in the ipsilateral lumbar spinal cord by day 10, reaching a low of about 40% of its normal value by day 20, and recovered to about 60% of its normal level by postoperative day 75. ChAc was the only protein among the four analyzed in this study in which changes in the ipsilateral lumbar spinal cord served as a reasonably good indicator of changes in ventral root activity. With the other three proteins, most of the detectable change occurred in the ventral root only.
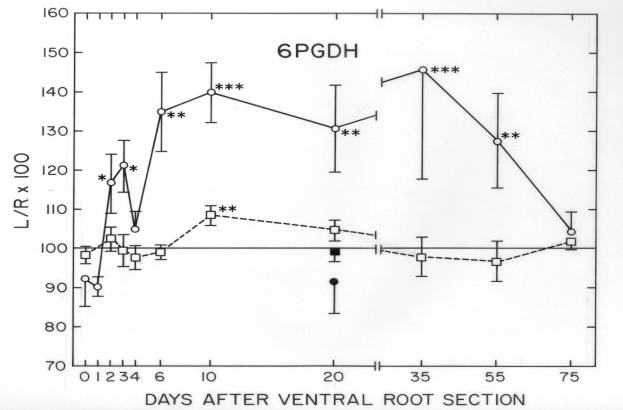
6-Phosphogluconate dehydrogenase (6-PGDH). The activity of this enzyme was measured by a fluorometric assay (Sinicropi and McIlwain,1983). By postoperative day 2, 6-PGDH was 20% higher in the injured proximal ventral root than on the uninjured side, rising to approximately 40% above normal values between days 10 and 35, and then returning to normal by day 75 ![]() . In the spinal cord, a 10% increase in its activity was observed only at postoperative day 10.
. In the spinal cord, a 10% increase in its activity was observed only at postoperative day 10.
Tubulin. 3H-colchicine binding was used as a measure of tubulin dimers in the ventral root and spinal cord of axotomized and control frogs (Sinicropi and McIlwain,1983). A 50% decline in tubulin content in the injured proximal ventral root was observed by day 20, remaining depressed until at least day 75. In contrast, no change in tubulin was detected on the ipsilateral side of the lumbar spinal cord during the 75 day postoperative period.
Overall, our data indicated that changes in these four proteins were usually greater in the proximal stump of the transected motor axons than in the spinal cord ipsilateral to the injury. A common feature of the axonal changes was that on postoperative day 3, all four proteins appeared to be increased in the proximal stump, although only two of them (AChE and 6-PGDH) showed statistically significant elevations. The interval to onset and duration of each change varied. For example, the most rapidly appearing change (AChE activity) was not the first to subside, and only two of the four proteins (AChE and 6-PGDH) had returned to normal values in the proximal axon by day 75.
2. How do these proteins change within axotomized spinal motor neuron cell bodies?
We examined the time course of changes in AChE in frog motor neuron cell bodies isolated as early as 8h after ventral root transection and as late as 20 days thereafter (Sinicropi et al., 1982). A slight, but statistically insignificant increase in AChE activity was observed between 8h and 48h after axotomy, followed by a decline in the activity of the enzyme in the injured cell bodies. The loss of AChE activity in the ipsilateral spinal cord 20 days after axotomy surpassed that measured in the isolated cell bodies, suggesting that the enzyme activity also decreased in other sites outside of the cell body and proximal dendrites. Overall, axotomy produces an early increase in AChE activity in the proximal stump, but little or no change in the cell body, while the activity of AChE declines in both the cell body and proximal axon as the response to injury continues.
The activity of 6-PGDH in the injured cell bodies was not measured at early time points after ventral root transection, but by postoperative day 20 it had increased significantly to about 90% above its average control level (Sinicropi and McIlwain, 1983). At that time its activity was also elevated in the proximal stump ![]() .
.
Technical problems prevented quantitative analyses of ChAc activity within the isolated frog motor neurons in these experiments.
Quantitative 2-dimensional polyacrylamide gel electrophoresis was used to detect changes in alpha– and beta -tubulin and in the 68Kd neurofilament protein in axotomized frog motor neuron cell bodies 20 days after ventral root transection (Sinicropi and McIlwain, 1983). The cell body content of each of these three proteins increased by between 40 and 90%, with the increases in a-tubulin and NF68 being statistically significant. Thus, at postoperative day 20, the content of these three cytoskeletal proteins was increased in the cell body, but depressed in the proximal axon.
3. How much does the total protein content of spinal motor neuron cell bodies increase after ventral root transection?
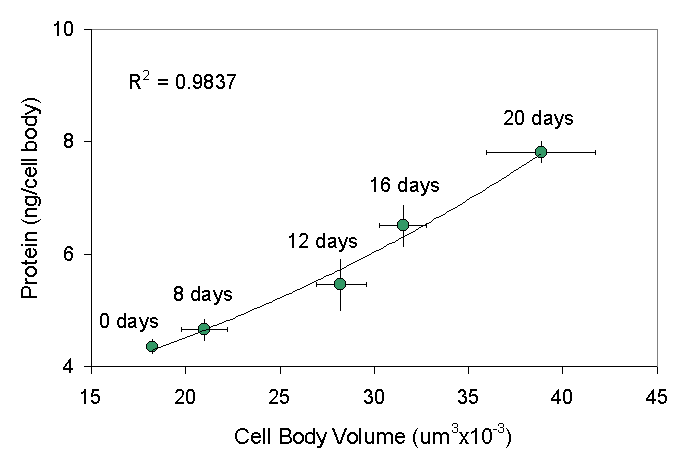
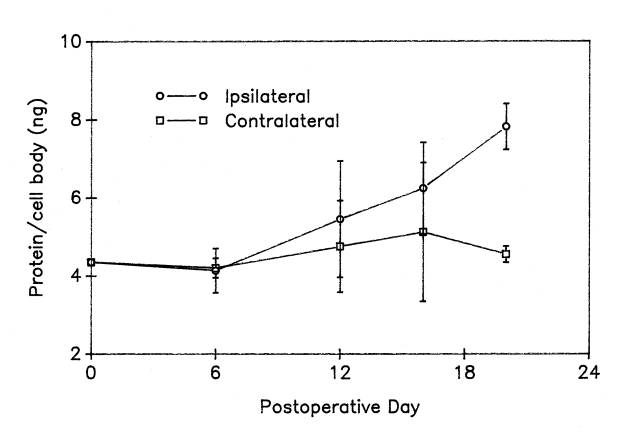
In a study of cell bodies isolated at different intervals up to 20 days after unilateral ventral root transection axotomy, we found a progressive increase in the total protein mass within the axotomized frog motor neuron cell bodies ![]() . After a delay of approximately 6 days, there is a nearly linear increase in total cell body protein within the injured motor neurons. On postoperative day 20, protein content was 7.82 ng/cell body, a significant increase of 80% over the normal value of 4.35 ng/cell body.
. After a delay of approximately 6 days, there is a nearly linear increase in total cell body protein within the injured motor neurons. On postoperative day 20, protein content was 7.82 ng/cell body, a significant increase of 80% over the normal value of 4.35 ng/cell body.
4. Is the increase in protein content a consequence of increased protein synthesis?
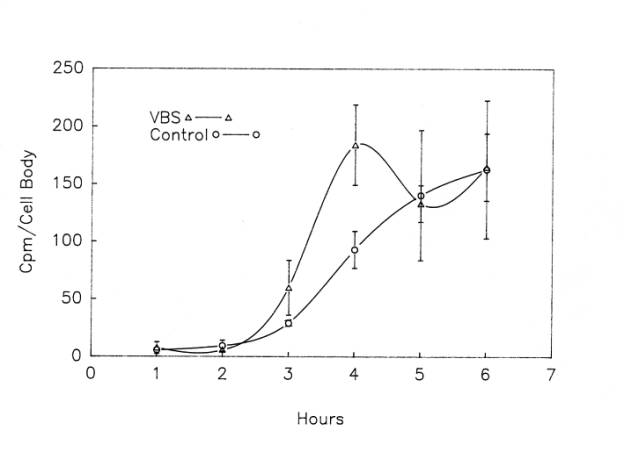
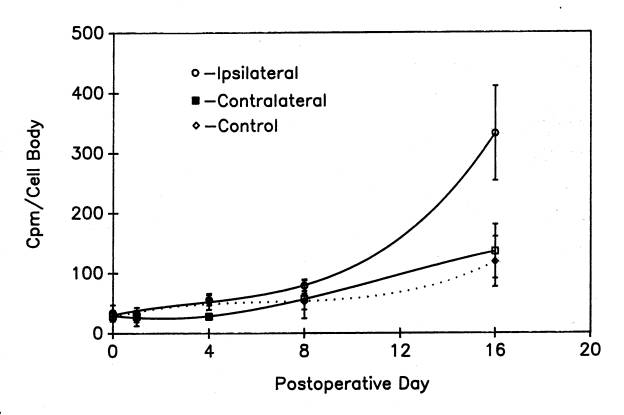
Although protein labeling experiments with radioactive amino acid precursors cannot distinguish among changes in synthesis, degradation and export, the absence of an increase in protein labeling would rule out increased synthesis as a cause of the increase in protein content in injured motor neurons. Thus, we applied our protein labeling protocol ![]() to injured frog lumbar motor neurons . Motor neuron cell bodies isolated from the side of the spinal cord ispilateral to ventral root transection showed a robust, non-linear increase in protein radiolabeling
to injured frog lumbar motor neurons . Motor neuron cell bodies isolated from the side of the spinal cord ispilateral to ventral root transection showed a robust, non-linear increase in protein radiolabeling ![]() , which by postoperative day 16 (the latest day analyzed in these experiments) was six times higher than in control motor neurons from unoperated frogs (McIlwain and Hoke, 2005). Note that the increase in protein labeling after axotomy far exceeded the 80% increase in total protein content in the injured cell bodies. Although these data do not rule out a decreased degradation or export of newly labeled protein, they strongly suggest an increase in the rate of total protein synthesis after axotomy when they are considered in the light of our findings on total RNA synthesis after axotomy.
, which by postoperative day 16 (the latest day analyzed in these experiments) was six times higher than in control motor neurons from unoperated frogs (McIlwain and Hoke, 2005). Note that the increase in protein labeling after axotomy far exceeded the 80% increase in total protein content in the injured cell bodies. Although these data do not rule out a decreased degradation or export of newly labeled protein, they strongly suggest an increase in the rate of total protein synthesis after axotomy when they are considered in the light of our findings on total RNA synthesis after axotomy.
5. How much is total RNA synthesis altered after ventral root transection?
Because motor neurons are capable of regenerating their axons after injury, it has long been thought that many of their responses to axonal injury are anabolic. An increase in total RNA synthesis appeared to be part of those responses, based upon a variety of different observations on axotomized motor neurons, including increased nucleolar volume (Lieberman, 1971), inhibition of morphological responses by actinomycin D (Torvik and Heding, 1969), an inhibitor of RNA synthesis, and increases in total cell body RNA (Edström, 1959) and RNA labeling (Watson, 1965). However, none of these experiments quantified RNA synthesis per se. As argued above for protein labeling, increased RNA labeling after axotomy could be attributed to increased synthesis, decreased degradation or export of RNA, or a combination of these factors.
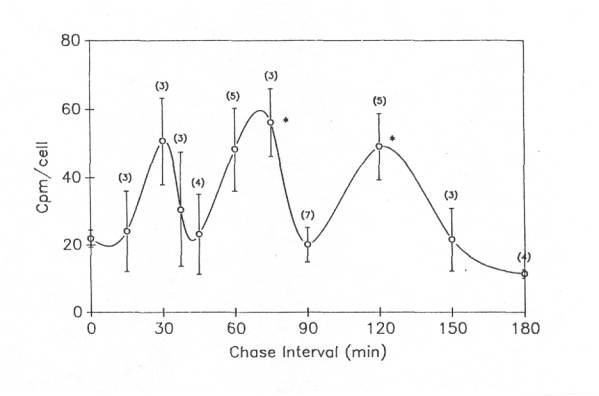
For a more definitive answer to whether the rate of RNA synthesis increases after axotomy, we used the nuclear run-on technique, mentioned above, which excludes RNA degradation and export and quantifies nascent RNA chains bound to DNA. We found that transcription did indeed increase in frog motor neuron cell bodies after axotomy (Burgess and McIlwain, 1994). The increases were multiphasic, with three separate bursts of RNA synthesis during the first 16 days after unilateral ventral root transection. At the peaks of the three events, total transcriptional activity was 2-5 times greater than in unoperated control motor neurons.
Unlike the RNA labeling results, the increases in total protein labeling and content were not multiphasic ![]() . Nonetheless, because total RNA and protein labeling as well as protein content are all increased after axotomy, an increase in the rate of total protein synthesis may well play a significant role in the accumulation of protein within the injured motor neuron cell body. Further discussion of this possibility is found in McIlwain and Hoke (2005). An increase in protein synthesis after axotomy would not exclude the possibility that other mechanisms (e.g., decreased rate of degradation or export of new protein) may also increase cell body protein content after axotomy, since there is reason to suspect that protein being exported to the injured axon may dam up within the cell body
. Nonetheless, because total RNA and protein labeling as well as protein content are all increased after axotomy, an increase in the rate of total protein synthesis may well play a significant role in the accumulation of protein within the injured motor neuron cell body. Further discussion of this possibility is found in McIlwain and Hoke (2005). An increase in protein synthesis after axotomy would not exclude the possibility that other mechanisms (e.g., decreased rate of degradation or export of new protein) may also increase cell body protein content after axotomy, since there is reason to suspect that protein being exported to the injured axon may dam up within the cell body ![]() .
.
6. Does DNA synthesis occur in injured motor neuron cell bodies?
Our studies on the DNA content of normal motor neurons ![]() did not rule out the possibility that larger spinal motor neurons may have somewhat more total DNA than small motor neurons. However, we have found no evidence for DNA synthesis in normal or axotomized motor neurons (McIlwain and Farel, 1979). In that study, we used the incorporation of 3H-thymidine to determine the time course of increased labeling of new DNA in the spinal cords of frogs whose motor axons had been unilaterally transected. In the same animals, we did find evidence for an increase in DNA labeling in nearby non-neuronal cells. Injury close to the cell body produced a larger increase in labeling in non-neuronal cells than did transection of the sciatic nerve. In each instance, a single peak of DNA labeling occurred over a 3-day period after injury. This result indicates that the signals that initiate increased DNA labeling in non-motor neurons after ventral root transection are not identical to those that produce the multiphasic increases in RNA synthesis in injured motor neurons
did not rule out the possibility that larger spinal motor neurons may have somewhat more total DNA than small motor neurons. However, we have found no evidence for DNA synthesis in normal or axotomized motor neurons (McIlwain and Farel, 1979). In that study, we used the incorporation of 3H-thymidine to determine the time course of increased labeling of new DNA in the spinal cords of frogs whose motor axons had been unilaterally transected. In the same animals, we did find evidence for an increase in DNA labeling in nearby non-neuronal cells. Injury close to the cell body produced a larger increase in labeling in non-neuronal cells than did transection of the sciatic nerve. In each instance, a single peak of DNA labeling occurred over a 3-day period after injury. This result indicates that the signals that initiate increased DNA labeling in non-motor neurons after ventral root transection are not identical to those that produce the multiphasic increases in RNA synthesis in injured motor neurons ![]() . In addition, we used autoradiography to ascertain that both glia and capillary endothelial cells showed increased 3H thymidine incorporation in all quadrants of the lumbar spinal cord after unilateral transection of ventral root 9. Unilateral dorsal root transection produced a different pattern of DNA labeling in non-neuronal cells, and was also seen on both sides of the lumbar spinal cord.
. In addition, we used autoradiography to ascertain that both glia and capillary endothelial cells showed increased 3H thymidine incorporation in all quadrants of the lumbar spinal cord after unilateral transection of ventral root 9. Unilateral dorsal root transection produced a different pattern of DNA labeling in non-neuronal cells, and was also seen on both sides of the lumbar spinal cord.
B. Motor Neuron Size After Injury: Morphological and Biochemical Relationships
1. What is the magnitude and time course of changes in nucleolar, nuclear and cell body size after axotomy?
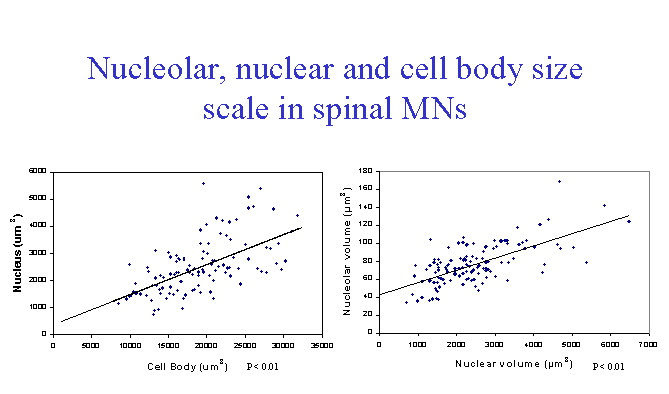
Unilateral transection of the lumbar ventral roots in the frog leads to an increase in the size of the nucleus, nucleolus and cell body of the injured motor neurons (Burgess and McIlwain, 1994). In the study just cited, computer-assisted measurements of nucleolar, nuclear and cell body area were made on isolated spinal motor neurons. The nucleolus showed the largest relative increase at 8, 12 and 16 days after injury, while the nucleus and cell body enlarged more slowly and to a lesser, but equal extent, relative to their normal sizes. Morphometric analyses were not continued until the injury-induced enlargements had begun to subside.
In a second study (McIlwain and Hoke, 1999), nucleolar, nuclear and cell body areas were quantified in fixed, sectioned lumbar spinal cord 7 days following lumbar spinal nerve transection. Changes similar to those observed on postoperative day 8 in isolated cell bodies were found, with the nucleolus showing the largest and the nucleus the smallest increase in area.
A third study (McIlwain and Hoke, 2005) quantified changes in volumes of the nucleolus, nucleus and cell body in lumbar motor neurons after ventral root transection. Lumbar motor neuron cell bodies showed the greatest increase in size in the nucleolus and the least change in the nucleus. Scaling of the nucleolus, nucleus and cell body was observed in the axotomized motor neurons in these animals, as in non-injured motor neurons. In none of our research was enlargement of the nucleolus, nucleus or cell body observed in non-injured lumbar or cervical motor neuron cell bodies. Direct injury to the axon appears to be required for their enlargement.
While nucleolar, nuclear and cell body size all change in the same direction in normal and axotomized animals, there is some autonomy in the control of the size of each of the three structures. Thus, in the frog, the rank order of relative increases in nucleolar, nuclear and cell body size after axotomy (nucleolus>cell body >nucleus) differs from that in normal motor neurons of increasing cell body size (cell body>nucleus>nucleolus). The functional significance of the shift in their rank order of increase after axotomy remains unclear.
2. Do large motor neurons undergo the same relative increases in nucleolar, nuclear and cell body size as small motor neurons?
We addressed this question by comparing frequency histograms of 7-day axotomized nucleolar, nuclear and cell body areas to those of unoperated control motor neurons, normalized by their mean increase in size (McIlwain and Hoke, 1999). We found that motor neurons of all sizes appeared to respond to axotomy with approximately the same percent increases in nucleolar, nuclear and cell body size. Thus, with respect to these particular morphological responses to injury, large motor neurons do not appear to be more reactive to axotomy than smaller motor neurons.
3. Are changes in the cytoskeleton after axotomy responsible for the conjugate enlargement of the nucleolus, nucleus and cell body?
Although these structural changes in injured motor neurons had been recognized and explored for over a century (Lieberman, 1971), no convincing explanation had been made for them. Suggestions that the entry of water causes enlargement of the injured cell body were consistent with decreases in RNA and protein concentrations observed for a few days after axotomy in rabbit hypoglossal motor neurons (Brattgård et al., 1957). However, these concentration changes did not persist as the hypoglossal motor neurons continued to enlarge, and they were not found in axotomized frog lumbar motor neurons (Edström, 1959).
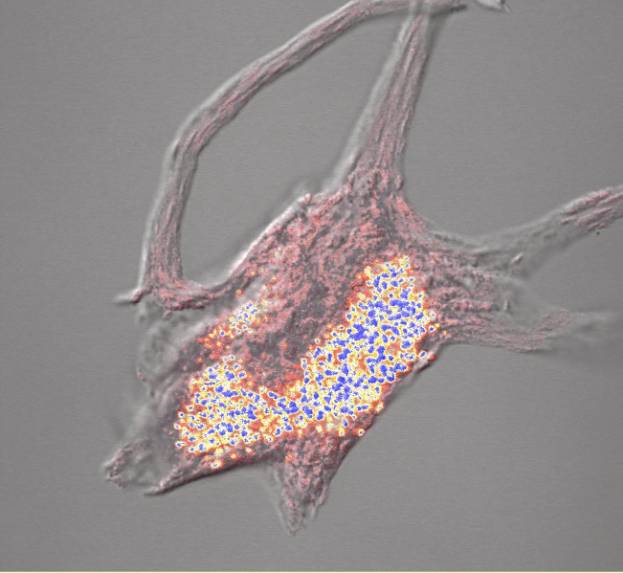
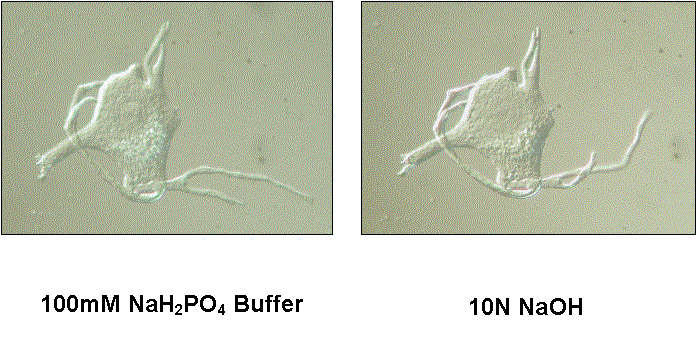
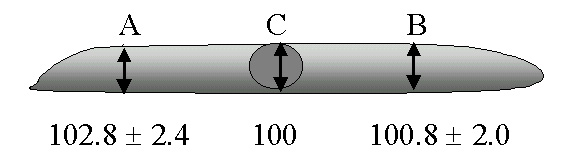
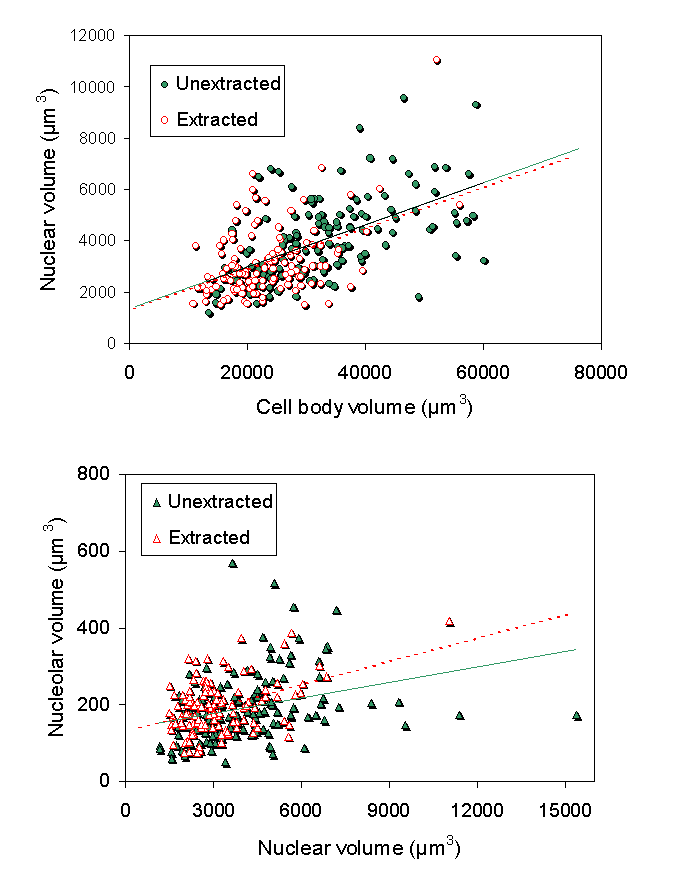
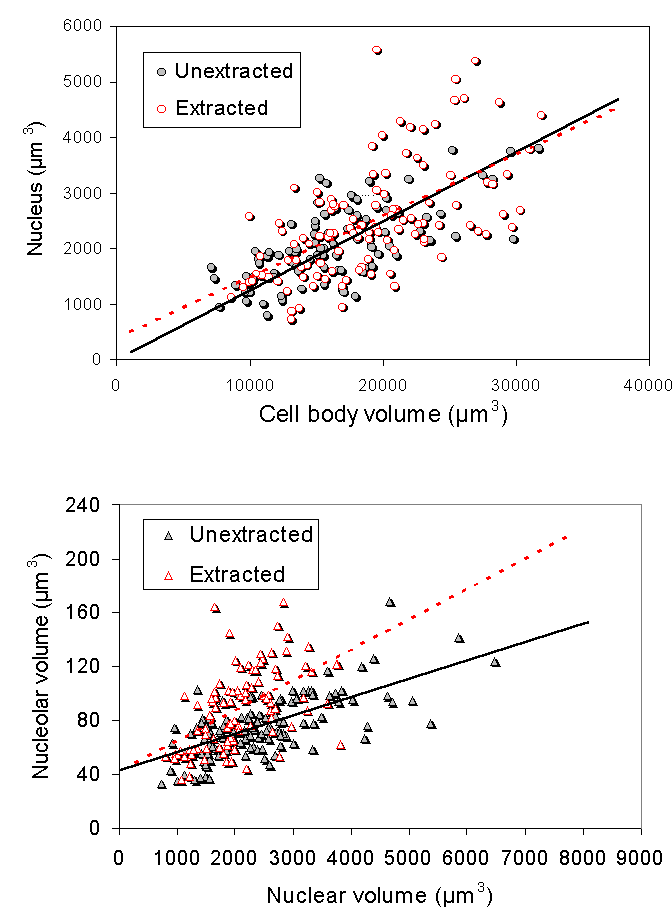
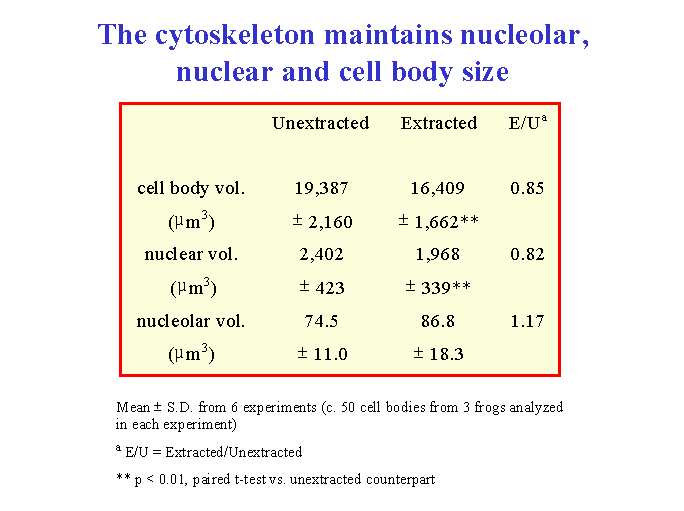
We explored a different hypothesis for these morphological changes. We investigated the role of the cytoskeleton in injury-induced morphological changes after axotomy, using the cell body extraction procedure previously described (McIlwain and Hoke, 2005). We found that the cytoskeleton of axotomized motor neuron cell bodies underwent nearly the same increases in mean nucleolar, nuclear and cell body volumes as observed in axotomized motor neurons that were not extracted ![]() . Scaling of the nucleolus, nucleus and cell body was also preserved in cytoskeletons isolated from axotomized motor neurons
. Scaling of the nucleolus, nucleus and cell body was also preserved in cytoskeletons isolated from axotomized motor neurons ![]() . Thus, we concluded that the cytoskeleton of normal motor neuron cell bodies maintains the nucleolar, nuclear and cell body size relationships in normal motor neurons and enlarges after axotomy in a manner commensurate with the changes seen in unextracted motor neurons.
. Thus, we concluded that the cytoskeleton of normal motor neuron cell bodies maintains the nucleolar, nuclear and cell body size relationships in normal motor neurons and enlarges after axotomy in a manner commensurate with the changes seen in unextracted motor neurons.
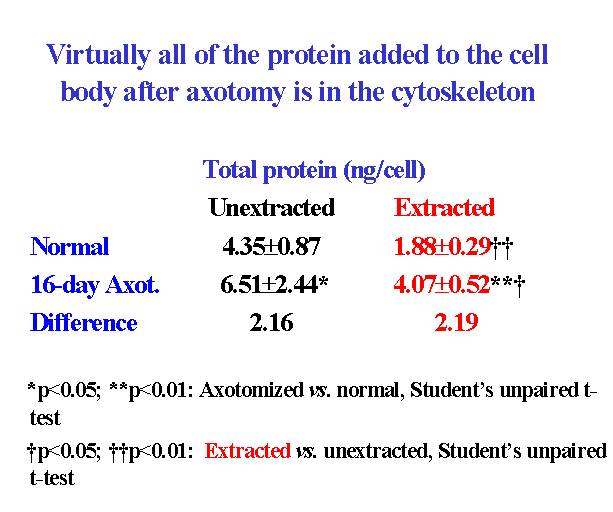
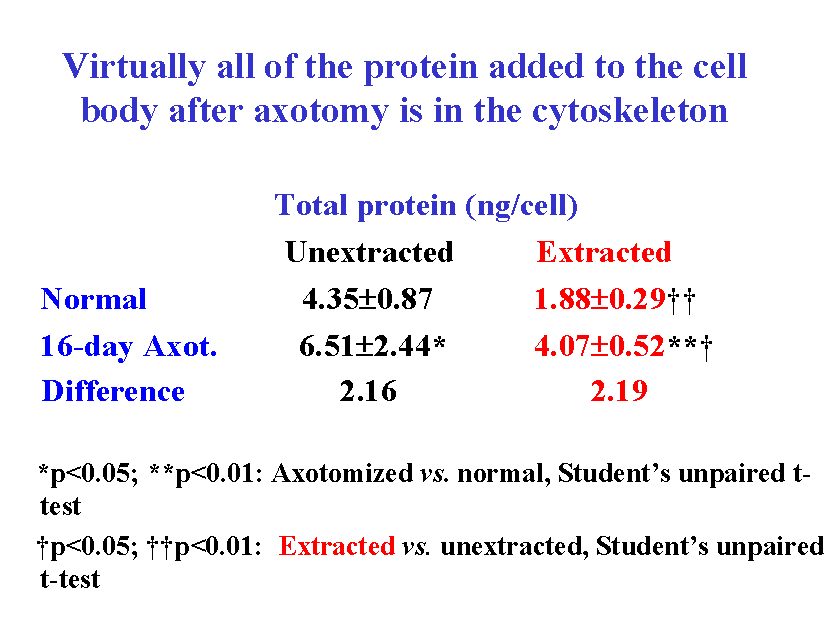
4. Is protein added to the cell body cytoskeleton after axotomy?
The progressive increase in cell body size in lumbar spinal motor neurons after axotomy is closely associated with an increase in total protein content ![]() . A comparison of the protein added to extracted and unextracted cell bodies 16 days after ventral root transection showed that virtually all of the protein added to the cell body after axotomy was associated with the cytoskeleton
. A comparison of the protein added to extracted and unextracted cell bodies 16 days after ventral root transection showed that virtually all of the protein added to the cell body after axotomy was associated with the cytoskeleton ![]() . We infer that the addition of protein to the cytoskeleton probably leads to the enlargement of the nucleolus, nucleus and cell body after axotomy.
. We infer that the addition of protein to the cytoskeleton probably leads to the enlargement of the nucleolus, nucleus and cell body after axotomy.
Furthermore, there is evidence that the protein added to the cytoskeleton is newly synthesized protein (McIlwain and Hoke, 2005). Although it is reasonable to suspect that the increase in total protein labeling after axotomy reflects an increased rate of cytoskeletal protein synthesis, it is still possible that a decrease in the rate of degradation or export of new cytoskeletal protein contributes to the increase in new protein in the cell body cytoskeleton. Predictably, not all of the newly synthesized protein in axotomized motor neurons is associated with the cytoskeleton. Only one-half of the six-fold increase in radiolabeled protein is directed to the cytoskeleton in cell bodies isolated 16 days after axotomy (McIlwain and Hoke, 2005). The other half of the newly synthesized protein does not contribute measureably to the total protein content of the cell body, but appears to be exported and/or degraded.
Thus, we propose that axotomy preferentially adds protein mass to the nucleocytoplasmic cytoskeleton, leading to the conjugate enlargement of the nucleolus, nucleus and cell body. A decrease in the rate of degradation of protein in the nucleocytoplasmic matrix could also play a role in the injury-induced enlargement of the nucleolus, nucleus and cell body. A possible role for a decrease in the rate of export of axonal cytoskeleton after axotomy is discussed below ![]() .
.
5. Does an increase in total transcription in spinal motor neurons always lead to increased nucleolar, nuclear and cell body size and protein content?
Increased nucleolar or nuclear size is sometimes used as an indication of increased RNA and protein synthesis (Aldskogius et al., 1980). We had an opportunity to test whether this is invariably true by comparing morphological and transcriptional analyses in injured and non-injured motor neurons within the same animal. In our study of transcriptional changes in motor neurons after unilateral transection of the lumbar ventral roots in frog (Burgess and McIlwain, 1994), we found that the multiphasic increases in RNA synthesis in axotomized lumbar motor neurons were also seen to a lesser extent in non-injured lumbar motor neurons on the contralateral side of the spinal cord. However, increases in nucleolar, nuclear and cell body area were found only in the injured motor neurons. Similarly, increased cell body protein content occurred only on the side of the injury ![]() . Thus, under these circumstances, transcriptional increases can be dissociated from increases in nucleolar, nuclear size and protein content. We conclude that enlargement of the nucleolus or nucleus after axotomy and increased cell body total protein are not obligatory consequences of increased transcription, but requires other factors as well.
. Thus, under these circumstances, transcriptional increases can be dissociated from increases in nucleolar, nuclear size and protein content. We conclude that enlargement of the nucleolus or nucleus after axotomy and increased cell body total protein are not obligatory consequences of increased transcription, but requires other factors as well.
6. Do changes in the cytoskeleton underlie increased nuclear eccentricity after axotomy?
The addition of new protein to the nucleocytoplasmic cytoskeleton after axotomy ![]() does not explain increased nuclear eccentricity. Based upon evidence that the cytoskeleton maintains nuclear location in non-neuronal cells (Hefland et al., 2003), we analyzed nuclear position in cytoskeletons isolated from non-injured, extracted motor neuron cell bodies, using the method of Barr and Hamilton (1948). Extracted frog motor neurons showed the same degree of nuclear eccentricity found in unextracted cell bodies
does not explain increased nuclear eccentricity. Based upon evidence that the cytoskeleton maintains nuclear location in non-neuronal cells (Hefland et al., 2003), we analyzed nuclear position in cytoskeletons isolated from non-injured, extracted motor neuron cell bodies, using the method of Barr and Hamilton (1948). Extracted frog motor neurons showed the same degree of nuclear eccentricity found in unextracted cell bodies ![]() (McIlwain and Hoke, 2005). Approximately 10% of these cell bodies, whether extracted or unextracted, had nuclei that contacted the cell periphery (100% eccentricity). After ventral root transection, nuclear eccentricity in the isolated cytoskeleton increased to the same degree as it did in unextracted cell bodies
(McIlwain and Hoke, 2005). Approximately 10% of these cell bodies, whether extracted or unextracted, had nuclei that contacted the cell periphery (100% eccentricity). After ventral root transection, nuclear eccentricity in the isolated cytoskeleton increased to the same degree as it did in unextracted cell bodies ![]() . Approximately 60% of the injured cell bodies had nuclei that touched the cell periphery, whether or not they were extracted. We concluded that the cytoskeleton maintains both nuclear position and nuclear size in normal motor neurons and that axotomy alters the location of the nucleus by restructuring the cytoskeleton. Since axotomy increases the frequency with which the nucleus makes contact with the cell periphery, protein must be asymmetrically removed, as well as added to the cytoskeleton as it enlarges.
. Approximately 60% of the injured cell bodies had nuclei that touched the cell periphery, whether or not they were extracted. We concluded that the cytoskeleton maintains both nuclear position and nuclear size in normal motor neurons and that axotomy alters the location of the nucleus by restructuring the cytoskeleton. Since axotomy increases the frequency with which the nucleus makes contact with the cell periphery, protein must be asymmetrically removed, as well as added to the cytoskeleton as it enlarges.
Other investigators have reported that the direction of increased nuclear eccentricity in axotomized motor neurons is away from the axon hillock (Lieberman, 1971). We could not determine the direction of increased eccentricity in isolated cell bodies, since the axon is lost during cell body isolation. Nevertheless, those reports, along with our finding of an overall expansion of the cytoskeleton after axotomy, indicate that the cytoskeleton enlarges between the nucleus and axon hillock.
If so, why would the cytoskeleton expand preferentially in that particular region of the cell? One possibility is that proteins associated with the axonal cytoskeleton dam up in that region of the cell body, because they are unable to enter the truncated axon as rapidly as they are being produced. It is easy to imagine a prolonged overproduction of axonal cytoskeletal proteins in motor neurons whose total transcription ![]() and protein content
and protein content ![]() increase for weeks following an abrupt loss of more than 90% of the cell’s total volume after ventral root transection.
increase for weeks following an abrupt loss of more than 90% of the cell’s total volume after ventral root transection.
7. Does damming up of axonal cytoskeleton cause increased nuclear eccentricity, chromatolysis and cell body enlargement after axotomy?
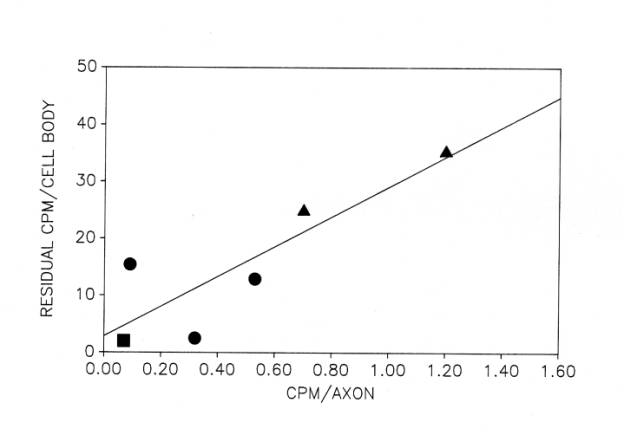
Prior to our studies on the cytoskeleton, we had noted the possibility that axonal constituents may dam up in the injured frog cell body (Sinicropi and McIlwain, 1983). That suggestion was based upon the observation that cytoskeletal proteins increase within the axotomized motor neuron cell body, but decrease in the proximal axon. During the period when the content of alpha– and beta-tubulin and NF68 cytoskeletal proteins was increasing within axotomized cell bodies ![]() , significant decreases were observed in their content in the ventral root proximal to the site of injury. These decreases, determined on postoperative day 35, were estimated to reflect changes in the content of these proteins first delivered to the axon on about day 20, based upon an anterograde transport rate of about 0.5mm per day. If the content of these three proteins is indeed increased in the cell body at a time when their delivery to the axon is decreasing, then an increase in their rate of synthesis could not solely account for both observations. It is possible, for instance, that some protein destined for the damaged axon is dammed up in the cell body.
, significant decreases were observed in their content in the ventral root proximal to the site of injury. These decreases, determined on postoperative day 35, were estimated to reflect changes in the content of these proteins first delivered to the axon on about day 20, based upon an anterograde transport rate of about 0.5mm per day. If the content of these three proteins is indeed increased in the cell body at a time when their delivery to the axon is decreasing, then an increase in their rate of synthesis could not solely account for both observations. It is possible, for instance, that some protein destined for the damaged axon is dammed up in the cell body.
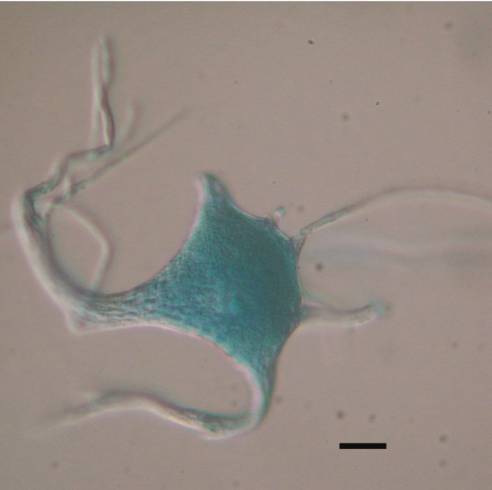
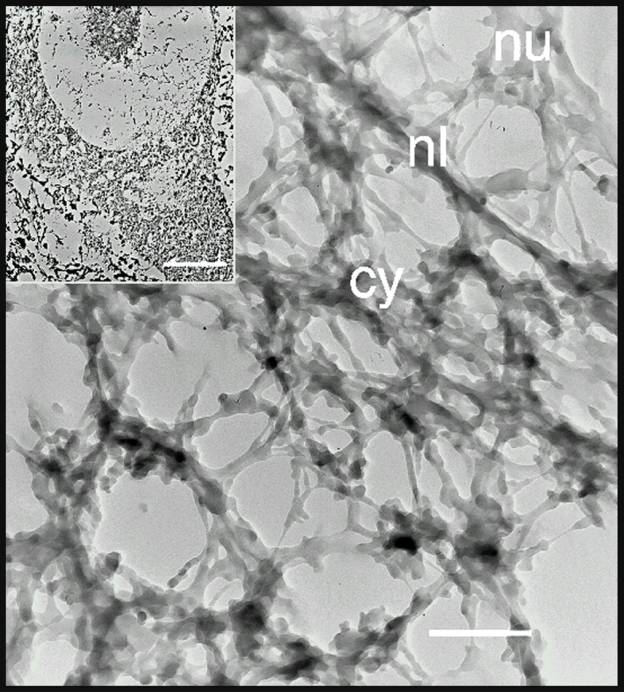
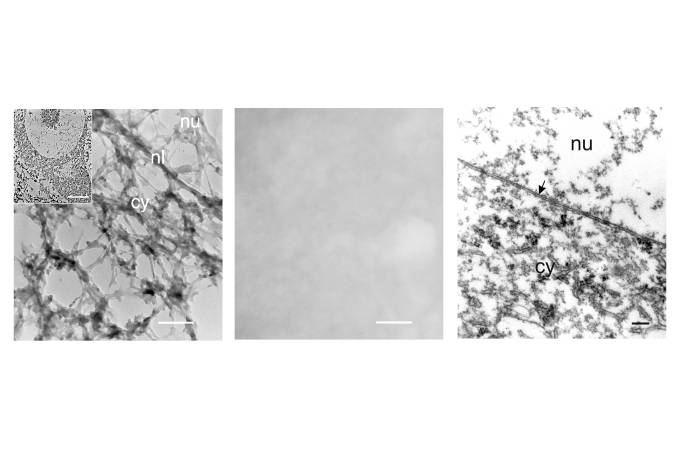
Another reason to suspect that cytoskeletal constituents of the axon may dam up within the injured cell body arose from our study on the isolated cytoskeleton of spinal motor neurons (McIlwain and Hoke, 2005). We found that Nissl bodies are associated with the isolated cytoskeleton of spinal motor neurons ![]() . Other investigators (Lieberman, 1971) had shown that chromatolysis, the disorganization of Nissl bodies and the consequent loss of basophilia that occurs after axotomy, begins in the region between the nucleus and axon hillock of spinal motor neurons. Two possible explanations for the appearance of chromatolysis in this region of the motor neuron are:
. Other investigators (Lieberman, 1971) had shown that chromatolysis, the disorganization of Nissl bodies and the consequent loss of basophilia that occurs after axotomy, begins in the region between the nucleus and axon hillock of spinal motor neurons. Two possible explanations for the appearance of chromatolysis in this region of the motor neuron are:
- Nissl bodies are removed from or no longer can be organized by cell body cytoskeleton that is added to that specific region of the cell.
- Cell body cytoskeleton containing Nissl bodies is displaced in that region by axonal cytoskeleton, which contains no Nissl bodies and which is unable to enter the truncated axon as rapidly as it is produced.
The second possibility provides a more readily understood reason for the development of chromatolysis specifically between the nucleus and axon hillock; namely, that Nissl body-free axonal cytoskeletal constituents dam up in the injured cell body. An accumulation of axonal cytoskeletal protein could contribute to the enlargement of the cell body. It also provides a plausible explanation for the shift in nuclear position away from the axon hillock. For this reason, we proposed (McIlwain and Hoke, 2005) that axotomy causes the axonal cytoskeleton to accumulate in the region between the nucleus and axon hillock, thereby increasing nuclear eccentricity in a direction away from the axon hillock and producing chromatolysis in that region of the injured motor neuron.
A requirement of the proposal is that it explain the direction in which chromatolysis develops. The loss of basophilic staining after axotomy is termed “central chromatolysis” because in many instances it first appears near the nucleus and spreads outward towards the axon hillock (Lieberman, 1971). If the axonal cytoskeleton dams up in this region of the injured cell body, why does the loss of basophilia spread centrifugally? It is not known exactly where assembly of the axonal cytoskeleton begins in normal or axotomized motor neurons. However, there is some information about the assembly of the cytoplasmic cytoskeleton in non-neuronal cells. In her pulse-chase analysis of the incorporation of newly synthesized proteins into the cytoskeletal framework of non-neuronal cells, Fulton (1984) provided autoradiographic evidence that new cytoskeleton is first assembled near the nucleus, and then moves towards the periphery of the cell. If the assembly of the axonal cytoskeleton in motor neurons follows the same pattern after axotomy, then chromatolysis might spread centrifugally towards the periphery. As the movement of newly synthesized axonal cytoskeleton towards the hillock is impeded, chromatolysis would appear first near the nucleus, spreading outward towards the hillock with time and displacing the basophilic cell body cytoskeleton. This action could produce a densely basophilic rim in the cell body periphery, as has often been observed (Lieberman, 1971). Further accumulation of the axonal cytoskeleton could ultimately involve other regions of the cell body.
A prediction of the proposal that axonal cytoskeleton dams up in the injured motor neuron is that the timing and magnitude of nuclear eccentricity and chromatolysis will closely parallel one another. For example, if the increase in nuclear eccentricity depends upon an accumulation of Nissl body-free cytoskeleton between the nucleus and axon hillock, one would expect that nuclear eccentricity would appear as or soon after chromatolysis begins and subside as basophilia returns. Lieberman (1971) has reviewed evidence supporting a temporal correlation between them, although he also cited several reports in which the two morphological responses were possibly dissociated.
Another prediction of this proposal is that the axonal cytoskeleton differs from the cytoplasmic cytoskeleton in ways that might be used to demonstrate the accumulation of axonal matrix between the nucleus and axon hillock. For instance, if one could identify a marker for the axonal cytoskeleton, not present in the cell body cytoskeleton, that marker could be used to visualize the axonal cytoskeleton in normal and axotomized motor neurons. A progressive accumulation of such a marker between the nucleus and axon hillock of axotomized motor neurons would support our hypothesis for the role of cytoskeletal changes in increased nuclear eccentricity and chromatolysis. What that marker might be is unclear, however, in view of uncertainties already alluded to about the composition of the cytoskeleton we isolate ![]() .
.
8. What signals the cell body that its axon has been injured?
In spinal motor neurons, the distance between a site of axonal injury and the motor neuron cell body can be great – potentially longer even than the length of an extremity. Somehow, the injury is signalled to the cell body, but the identity and route of the signal(s) have been elusive. We have considered three questions related to this issue:
a. Do proteins that accumulate proximal to the site of axonal injury return to the cell body and initiate the cell body’s responses to axotomy?
Biochemical changes in axotomized frog cell bodies begin to occur at least by 24h after axonal injury (Cerf and Chacko, 1958) and morphological changes appear by day 4 (Price and Porter, 1972). We explored this time frame, using AChE, an easily assayed enzyme that is rapidly transported in the motor axon (Sinicropi et al., 1982). During the first 3 days after ventral root transection, AChE activity accumulates 3-fold at the site of injury ![]() . However, when AChE activity was measured in the injured cell bodies at 8h, 1, 2 or 3 days after axotomy, no significant change was detected in its activity relative to contralateral controls or to unoperated controls. Since there is no significant change in the size of the frog bodies this soon after axotomy, we concluded that reflux of AChE from the axon was not a likely signal, unless only very small changes, undetected by our assays, are required for signalling.
. However, when AChE activity was measured in the injured cell bodies at 8h, 1, 2 or 3 days after axotomy, no significant change was detected in its activity relative to contralateral controls or to unoperated controls. Since there is no significant change in the size of the frog bodies this soon after axotomy, we concluded that reflux of AChE from the axon was not a likely signal, unless only very small changes, undetected by our assays, are required for signalling.
b. Are there systemic injury signals?
In addition to an axonally-mediated injury signal, it is possible that molecules arising from the injured axon or from nearby cells, such as Schwann cells, fibroblasts or invading macrophages, could be delivered to the circulation and reach the cell body. Evidence supporting this possibility was uncovered in our study on changes in motor neuron transcriptional activity after axotomy (Burgess and McIlwain, 1994). In addition to our finding multiphasic transcriptional changes both in injured and in contralateral, non-injured motor neurons, multiphasic transcriptional changes were also detected in uninjured cervical motor neurons. That injury-induced transcriptional changes having the same time course of those observed in the injured cell bodies would occur at such a distance from the lumbar motor neurons strongly suggested a systemic route for the signal(s). In a review article (Farel and McIlwain, 2000), this evidence, as well as data involving injury-induced neuron number changes, was used to propose a systemic route for unidentified signals arising from the injury site.
The presence of similar transcriptional changes in contralateral, non-injured motor neurons following unilateral lumbar ventral root transection indicates that they were initiated by the same injury signal(s) as cervical motor neurons. However, it is also possible that other signals are also involved in injury-induced changes in contralateral motor neurons and in non-neuronal cells (McIlwain and Farel, 1979) in the vicinity of the axotomized cell bodies.
c. Is the distal stump the source of injury signals?
The degenerating nerve distal to the site of injury is a plausible source of signals for the axon reaction. We looked for evidence of a dependence of nucleolar, nuclear and cell body enlargement upon the presence of the distal stump from frog lumbar spinal nerves (McIlwain and Hoke, 1999), but found none. Their conjugate enlargement after unilateral spinal nerve transection was the same, whether the most of the distal stump was removed from the animal, left in place, or transplanted to skin near a dorsal lumbar laminectomy, where access of putative soluble signals to motor neuron cell bodies might be enhanced. As with unilateral ventral root transection only, there was also no sign of enlargement in contralateral lumbar motor neurons.
9. Do motor neurons on the opposite side of the spinal cord from a unilateral ventral root injury respond to axotomy?
Throughout our investigations on axotomized frog motor neurons, the only contralateral changes we detected in non-injured motor neuron cell bodies were the impressive multiphasic transcriptional responses described above ![]() . In addition, we found changes in the labeling of DNA in non-neuronal cells on the contralateral side of the spinal cord
. In addition, we found changes in the labeling of DNA in non-neuronal cells on the contralateral side of the spinal cord ![]() . We detected no changes in nucleolar, nuclear or cell body size in the non-injured motor neurons (Burgess and McIlwain, 1994) and no significant changes in the amount of seaonally-adjusted total protein in motor neuron cell bodies isolated from the contralateral lumbar spinal cord
. We detected no changes in nucleolar, nuclear or cell body size in the non-injured motor neurons (Burgess and McIlwain, 1994) and no significant changes in the amount of seaonally-adjusted total protein in motor neuron cell bodies isolated from the contralateral lumbar spinal cord ![]() . Compared with unoperated controls, there was no increase in protein labeling in motor neuron cell bodies isolated from the contralateral spinal cord, when seasonal variations in labeling were controlled
. Compared with unoperated controls, there was no increase in protein labeling in motor neuron cell bodies isolated from the contralateral spinal cord, when seasonal variations in labeling were controlled ![]() .
.