Research Highlights
X-Ray Crystallography
 Alleviating cancer-drug toxicity: The colon cancer chemotherapeutic CPT-11 causes severe diarrhea due gut bacterial beta-glucuronidases that reactivate the drug. To target these enzymes without killing beneficial intestinal bacteria, we identified potent inhibitors by high-throughput screening that have no effect on the endogenous human enzyme, nor do they kill bacteria or harm mammalian cells. Crystal structures established that inhibitor selectivity is based on a loop unique to bacterial beta-glucuronidases (magenta residues in picture). Oral administration of an inhibitor protected mice from CPT-11-induced toxicity. We showed that drugs may be designed to inhibit undesirable enzyme activities in essential microbial symbiotes to enhance chemotherapeutic efficacy (Wallace, B.D. et al., Science, 2010).
Alleviating cancer-drug toxicity: The colon cancer chemotherapeutic CPT-11 causes severe diarrhea due gut bacterial beta-glucuronidases that reactivate the drug. To target these enzymes without killing beneficial intestinal bacteria, we identified potent inhibitors by high-throughput screening that have no effect on the endogenous human enzyme, nor do they kill bacteria or harm mammalian cells. Crystal structures established that inhibitor selectivity is based on a loop unique to bacterial beta-glucuronidases (magenta residues in picture). Oral administration of an inhibitor protected mice from CPT-11-induced toxicity. We showed that drugs may be designed to inhibit undesirable enzyme activities in essential microbial symbiotes to enhance chemotherapeutic efficacy (Wallace, B.D. et al., Science, 2010).
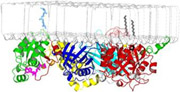 PLCs at membranes: Phospholipase C isozymes (PLCs) hydrolyze a minor phospholipid whose two products initiate bifurcating signaling cascades required for numerous cellular processes. The crystal structure of Rac1 bound to PLC-b2 and depicted here docked to a model membrane highlights the activation of PLC-b2 by Rac1 (Jezyk, M., Nat. Struct. & Mol. Biol., 2006).
PLCs at membranes: Phospholipase C isozymes (PLCs) hydrolyze a minor phospholipid whose two products initiate bifurcating signaling cascades required for numerous cellular processes. The crystal structure of Rac1 bound to PLC-b2 and depicted here docked to a model membrane highlights the activation of PLC-b2 by Rac1 (Jezyk, M., Nat. Struct. & Mol. Biol., 2006).
 Complex of RGS9/Gb5: Drugs that target G protein-coupled receptors (GPCRs) represent the largest class of clinically relevant therapeutics. Gbg dimers are obligate heterodimers needed for signaling propagated downstream of GPRCs. The crystal structure of the Gb5 subunit bound to RGS9 (depicted above) highlights an evolutionary distant Gbg-like complex required for signaling downstream of GPCRs in vision and neurotransmission (Cheever, M., Nat. Struct. Mol. Biol., 2008).
Complex of RGS9/Gb5: Drugs that target G protein-coupled receptors (GPCRs) represent the largest class of clinically relevant therapeutics. Gbg dimers are obligate heterodimers needed for signaling propagated downstream of GPRCs. The crystal structure of the Gb5 subunit bound to RGS9 (depicted above) highlights an evolutionary distant Gbg-like complex required for signaling downstream of GPCRs in vision and neurotransmission (Cheever, M., Nat. Struct. Mol. Biol., 2008).
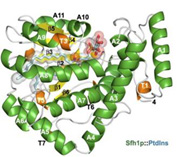 Phosphoinositide Homeostasis: Phosphorylated derivatives of phosphatidylinositol (PtdIns), termed phosphoinositides (PIPs) are important components of membrane associated signaling systems in eukaryotes. Crystal structures of Sfh1, a Ptdins/PtdCho-transfer protein, in complex with various phospholipids demonstrate how these ligands are bound and suggest how they are presented/transferred to kinases (Schaaf, G., Mol. Cell, 2008).
Phosphoinositide Homeostasis: Phosphorylated derivatives of phosphatidylinositol (PtdIns), termed phosphoinositides (PIPs) are important components of membrane associated signaling systems in eukaryotes. Crystal structures of Sfh1, a Ptdins/PtdCho-transfer protein, in complex with various phospholipids demonstrate how these ligands are bound and suggest how they are presented/transferred to kinases (Schaaf, G., Mol. Cell, 2008).
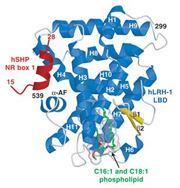 Structure determination of the human nuclear receptor LRH-1: This enzyme is critical to breast cancer development as it controls the expression of the aromatase enzyme required for estrogen-dependent tumor growth. Using structural and molecular biology, as well as mass spectrometry, we have shown that human LRH-1 is ligand-dependent and appears to respond to endogenous phospholipids that bind deep within the core of this transcription factor (Ortlund, E.A.,Nat. Struct. Mol. Biol., 2005).
Structure determination of the human nuclear receptor LRH-1: This enzyme is critical to breast cancer development as it controls the expression of the aromatase enzyme required for estrogen-dependent tumor growth. Using structural and molecular biology, as well as mass spectrometry, we have shown that human LRH-1 is ligand-dependent and appears to respond to endogenous phospholipids that bind deep within the core of this transcription factor (Ortlund, E.A.,Nat. Struct. Mol. Biol., 2005).
 General regulation of Phospholipase C (PLC):PLCs are enzymes involved in cell signaling. Aberrations in PLCs are reported to have an effect on malignant characteristics of tumors, such as motility and invasion capability. It has been suggested that PLC-ß2 constitutes a molecular marker of breast cancer cells able to monitor the progression to invasive cancers, and that it is a target for novel therapeutic approaches for breast cancer. Based on crystallographic and biophysical studies, we have recently proposed a general mechanism for the regulated auto-inhibition and activation of the entire family of phospholipase C (PLC) isozymes. This work is highlighted on the cover of Molecular Cell (Hicks, S., Mol. Cell, 2008).
General regulation of Phospholipase C (PLC):PLCs are enzymes involved in cell signaling. Aberrations in PLCs are reported to have an effect on malignant characteristics of tumors, such as motility and invasion capability. It has been suggested that PLC-ß2 constitutes a molecular marker of breast cancer cells able to monitor the progression to invasive cancers, and that it is a target for novel therapeutic approaches for breast cancer. Based on crystallographic and biophysical studies, we have recently proposed a general mechanism for the regulated auto-inhibition and activation of the entire family of phospholipase C (PLC) isozymes. This work is highlighted on the cover of Molecular Cell (Hicks, S., Mol. Cell, 2008).