Investigators: Support and Timeline

CWHR has a solid industry-sponsored trials process that provides reliable services with just enough room for customization depending on the project. The Center’s support is comprehensive and geared towards ensuring researchers and their departments maximize their return on time and resources. Services include:
- Navigating the Office of Clinical Trials approval process
- Establishing cost of protocol
- Budget negotiations
- Sponsor and study vetting
- Recruitment and enrollment tracking
- Monitoring financial health of trials.
Getting Started
PIs that wish to pursue a clinical trial through the CWHR should complete the Clinical Trial Support Request Form. You will be contacted within three business days by the CWHR Clinical Trial Manager to schedule a brief meeting to answer any questions, discuss the process, and clarify roles. CWHR will also supply a checklist to help evaluate whether or not a sponsor, and their clinical trial, are a good fit for the PI.
Timeline
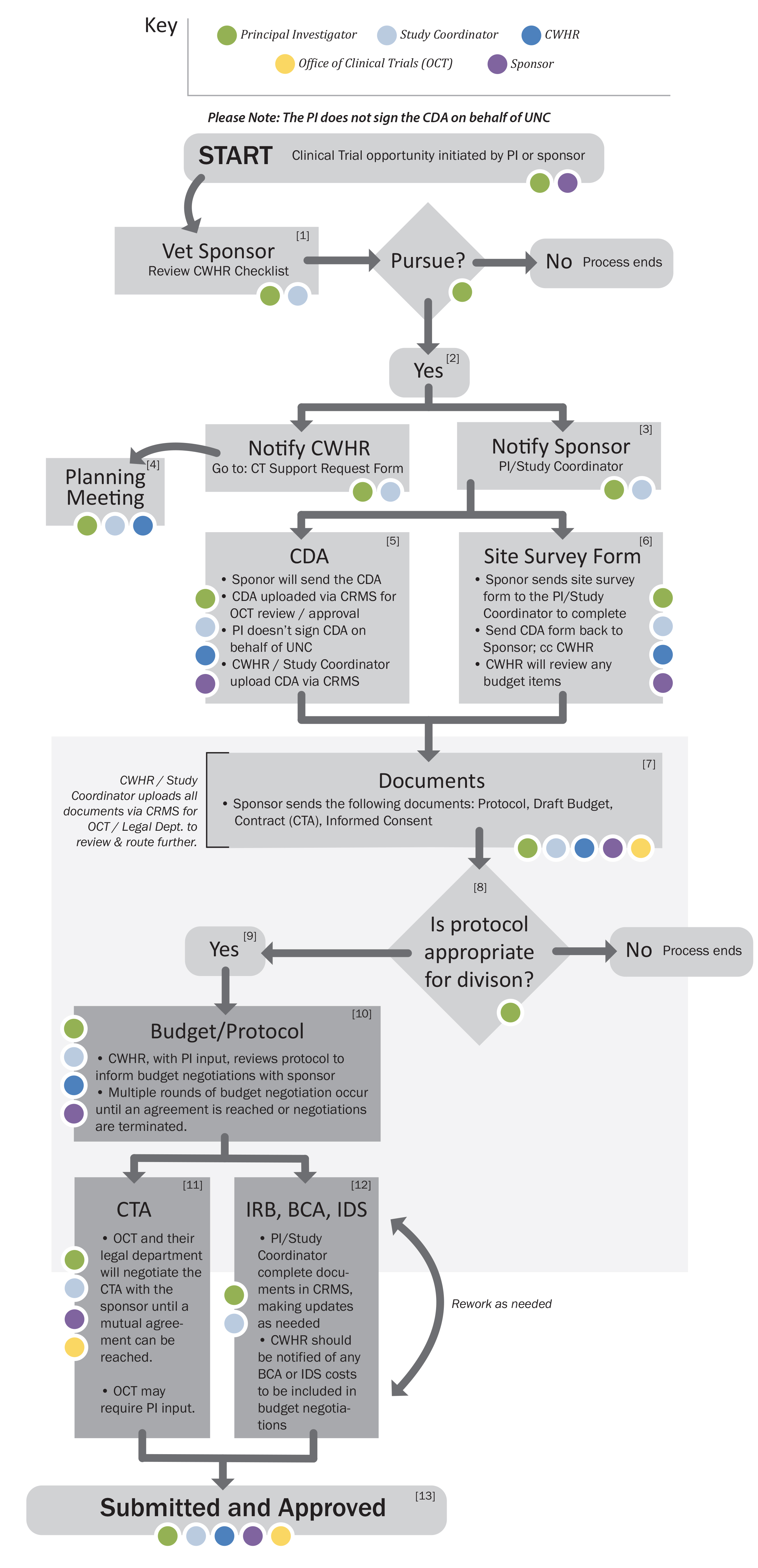
Clinical Trail processing is completed electronically via the Clinical Research Management System (CRMS). All numbers in brackets correspond to an action on the timeline. Colored circles refer to key players at each step.
(Narrative below)
Timeline Narrative
Step 1 – Decisions and Obtaining Documents
Decisions
[1] The decision to pursue an industry-sponsored research trial may be either PI or sponsor initiated. Either before or after the PI decides whether they would like to work with a sponsor, they may reach out to CWHR via the Clinical Trial Support Request Form. The Center can provide guidance based on experience and offer some considerations.
[2] If the PI ultimately decides that the project is one they would like to pursue, they should notify the sponsor.
[3] At that time the sponsor will obtain some basic information.
[4] CWHR will then conduct a planning meeting with the PI and, if there is one, their study coordinator. CWHR will also ensure the project is listed in CRMS.
CDA and Site Survey
[5] After the initial contact, the sponsor will forward a Confidential Disclosure Agreement (CDA) and Site Survey for completion. You are not authorized to sign the CDA on behalf of the university (see Pl Overall Responsibilities OSR Policy 200.4 and Departmental Administrator Responsibilities OSR Policy 200.5). The CDA, which is uploaded via CRMS by CWHR or the study coordinator, must be reviewed and approved by the Office of Clinical Trials (OCT) before UNC officials sign it. When CRMS routing has begun, either CWHR or the study coordinator will receive an automated email notifying OCT staff assigned to the account. OCT will review CDA (includes a review by their legal department), and troubleshoot any areas that they disagree with. Once approved and signed, OCT will send the CDA to the sponsor. Please allow two to four weeks for completion of this process.
[6] While the CDA is being reviewed, the PI will complete the site survey and return it directly to the sponsor.
Step 2 – Completing Documents
[7] Once the sponsor has the site survey and the signed CDA, they will send the PI a packet of regulatory documents; the packet will contain the following:
- Protocol
- Draft contract agreement
- Draft consent form
- Draft budget
- Other regulatory forms or documents
CWHR or the study coordinator will upload all documents via CRMS for routing to OCT.
First Priority – Protocol / Budget Review
[8] The PI’s initial focus should be on the protocol and budget. The Pl and his/her division should assess the value of the protocol and its feasibility per their own internal process. Consultation with others may occur as it is deemed appropriate by the division. At this point, the PI and the division will make a decision on whether or not to proceed. If the decision is made to not move ahead than the process ends here.
[9] If the decision is made to move forward, CWHR, as the fund administrator, will begin drafting a budget for negotiations.
Protocol / Budget Process
[10] The Pl or the study coordinator breaks the protocol into its component parts and obtains a cost for each component.
- Expenses are totaled to arrive at a per-patient cost to conduct the study.
- Staff salaries, start-up dollars, and IDS/BCA fees, etc. must be added to the budget as well as appropriate F&A to make sure that the payments will cover costs.
- It will also be important to carefully consider how long it will take to recruit subjects will last as this will inform the study period and how long salaries will need to be funded
- CWHR will assist with the budgeting calculation and process. If CWHR does not already have the sponsor’s proposed budget a copy will be obtained from the sponsor and compared to the estimated costs.
- There are a number of invoice-able costs that should be considered. CWHR will help you to consider these additional items.
- Very seldom does the sponsor’s proposed initial budget cover the actual cost of conducting the study, therefore, a period of negotiation follows. CWHR will assist with budget negotiations or will conduct them on the PI’s behalf with both their input and agreement.
- If a satisfactory budget agreement between the site and the sponsor cannot be reached, the PI should consider ending negotiations and the process as the study will not be financially viable. If this happens, OCT and CWHR must be notified of the decision.
The final agreed-upon budget is the second part of the final compilation package. The budget negotiation process can take from several weeks to several months, depending on how flexible the sponsor is and how accessible they are.
Simultaneous Processes
Two different processes will then occur simultaneously:
- Clinical Trial Agreement (CTA) review and negotiation
- Institutional Review Board (IRB), Billing Coverage Analysis (BCA), and Investigational Drug Service (IDS) are reviewed and reworked as needed
The CTA Approval Process
[11] The CTA, which has been uploaded via CRMS by this point, will be reviewed by the Office of Clinical Trials and their legal department. Negotiations and review will occur until OCT, the legal department, and the sponsor can come to a final agreement. The PI is able to provide input during this stage. The Pl and others such as CWHR personnel, division directors, department chairs, and/or colleagues should read the contract and provide OCT any points that they want to ensure are included. The contract spells out the legal arrangement of the partnership with the sponsor, indicates what triggers a payment, and, among other items, defines a payment schedule; the contract may also include budget information. Contract negotiations can take from several weeks to several months to complete, depending on the workload of OCT, the complexity of the contract, how easy the company is to deal with, and a multitude of other factors.
IRB, BCA, IDS Process
[12] The PI or study coordinator is responsible for completing all IRB, BCA and IDS documents via CRMS. BCA / IDS are completed as needed per study. Please notify CWHR of IDS / BCA costs to be incorporated into budget negotiations. As the IRB is reviewed, the PI or study coordinator work to resolve any issues that arise, and resubmit the application for approval. The IRB approval process can take from several weeks to several months depending on the timing of IRB meetings, how many issues they have with the protocol, and how thoroughly and timely the Pl (or designee) responds to those issues. The completed IRB is the third document in the final compilation package.
Finalized Documents
[13] Once the budget has been completed, it will be entered into RAMSeS by CWHR and routed for approvals. A project ID number is issued (Chartfield string) and CWHR prepares to administer the study. After the study has been routed and all approvals are received inRAMSeS, the Pl is notified and the final contract is executed.
Evaluating Study Health
Once the study is underway, the study coordinator will monitor recruitment and enrollment numbers. At the three-month mark, CWHR will conduct an in-depth review of the financial health of the study. If the study is not financially beneficial CWHR will work with the PI to remediate the situation. An additional three months will be given to see if the financial health of the study improves. If it does not demonstrate positive progress, CWHR will work to end the study according to the terms of the CTA unless the PI and/or their department/division agrees to cover all losses, including the administrative time required to administer the trial funds.