In-Home HIV Testing: Could You Be a Little More Sensitive, Please?
June 18, 2012
Guest Poster: Christopher Hurt, MD – Assistant Professor, Division of Infectious Diseases, The University of North Carolina – Chapel Hill
 This month we have a guest poster to our site, Dr. Christopher Hurt. Here at UNC, Dr. Hurt is considered a thoughtful and careful thinker about many of the most pressing contemporary issues in communicable diseases. His expertise ranges from transmission of drug-resistant HIV, analysis of sexual networks of HIV transmission, use of pre-exposure prophylaxis to prevent HIV, HCV treatment and novel approaches to influenza therapeutics. Here he shares his thoughts on home-based HIV testing. – DW
This month we have a guest poster to our site, Dr. Christopher Hurt. Here at UNC, Dr. Hurt is considered a thoughtful and careful thinker about many of the most pressing contemporary issues in communicable diseases. His expertise ranges from transmission of drug-resistant HIV, analysis of sexual networks of HIV transmission, use of pre-exposure prophylaxis to prevent HIV, HCV treatment and novel approaches to influenza therapeutics. Here he shares his thoughts on home-based HIV testing. – DW
Many of you probably heard the buzz in the media last month about at-home HIV testing. On May 15 2012, the FDA’s Blood Products Advisory Committee (BPAC) voted unanimously to recommend approval of an in-home HIV testing kit manufactured by OraSure Technologies. The kit is essentially the same as the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test, with which many of us are familiar from rapid testing in our clinics or at outreach testing events. Oral fluid wicks into an absorbent pad placed between the cheek and gum for several minutes, and then the whole device is set upright in a vial of liquid developer. Within 20 minutes, the user gets their result: one bar, negative; two, you’re HIV-positive. Many people don’t realize that a self-collection HIV test kit using dried blood spots has been commercially available in pharmacies since the FDA approved the method in the mid-1990s. Users prick their finger with a sterile lancet (like those used by diabetics), soak up the small droplet of blood onto a special card, and then mail the card in to a lab for antibody testing. Results are available within several days, by phone or online. Proponents of home OraQuick feel that it offers a distinct advantage over the existing lab-based method: you don’t have to wait for your results.
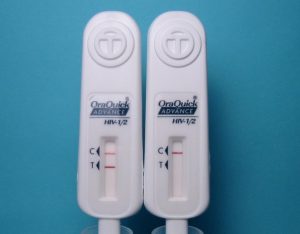 However, in studies conducted by the company leading up to the BPAC hearing, the sensitivity of the test in the hands of untrained users from a high prevalence (2%) population was only 92.9%, a substantial drop from the performance among trained personnel, where sensitivity was 99.3%. Let’s put it another way, hopefully to make it clearer: consider 1000 people who actually have established, antibody-positive HIV infection and are members of a population with a heavy burden of HIV already – like young, Black men who have sex with men (MSM), for example. If you gave the OraQuick at-home test kit to those 1000 people, only 929 would be correctly diagnosed (“true positives”) – leaving 71 incorrectly thinking they’re HIV-uninfected (“false negatives”). The exact same test, when used by trained personnel, would correctly identify 993 of those 1000, leaving only 7 false negatives. When the FDA modeled the data, they found that the number of false negatives we can expect per year with the at-home OraQuick test could be as high as 7,000. Additionally, the number of false positives – people who will be incorrectly diagnosed as having HIV when they really don’t – could reach as high as 3,600 per year. In their briefing document, the FDA summed up the conundrum: “[t]here is considerable personal and public health value in informing infected, but otherwise untested, persons of their true positive HIV status. However, this benefit is offset in some measure by HIV-positive individuals who receive an incorrect message that they are not infected (false negatives).” The pros outweighed the cons, and the committee voted 17-0 to recommend approval of the kit.
However, in studies conducted by the company leading up to the BPAC hearing, the sensitivity of the test in the hands of untrained users from a high prevalence (2%) population was only 92.9%, a substantial drop from the performance among trained personnel, where sensitivity was 99.3%. Let’s put it another way, hopefully to make it clearer: consider 1000 people who actually have established, antibody-positive HIV infection and are members of a population with a heavy burden of HIV already – like young, Black men who have sex with men (MSM), for example. If you gave the OraQuick at-home test kit to those 1000 people, only 929 would be correctly diagnosed (“true positives”) – leaving 71 incorrectly thinking they’re HIV-uninfected (“false negatives”). The exact same test, when used by trained personnel, would correctly identify 993 of those 1000, leaving only 7 false negatives. When the FDA modeled the data, they found that the number of false negatives we can expect per year with the at-home OraQuick test could be as high as 7,000. Additionally, the number of false positives – people who will be incorrectly diagnosed as having HIV when they really don’t – could reach as high as 3,600 per year. In their briefing document, the FDA summed up the conundrum: “[t]here is considerable personal and public health value in informing infected, but otherwise untested, persons of their true positive HIV status. However, this benefit is offset in some measure by HIV-positive individuals who receive an incorrect message that they are not infected (false negatives).” The pros outweighed the cons, and the committee voted 17-0 to recommend approval of the kit.
I agree in principle with the BPAC’s stance on approving in-home rapid tests, but I also have qualms about their use. First, we’ve been rightfully focused over the past several years on a “test, link, and treat” model of HIV prevention, given the potential impact of getting HIV-infected people onto therapy – both for individual and public health benefits. Yet, in-home rapid testing fulfills only the first part of this model, leaving the linkage part entirely up to the user. My worry is that it’s already difficult enough for newly diagnosed patients to get appointments set up with the assistance of counselors at testing centers or bridging case managers. How can we expect people who choose to test outside of the existing system to then seamlessly enter and navigate that system on their own? I do think there’s a happy medium between rapid tests and the current system of presenting to a testing center: testing of self-collected dried blood spot or oral fluid specimens in professional labs, with reporting and post-test counseling in person, online, or by phone. Such centralized testing has certain advantages, perhaps the most important of which is quality control. If conducted by public health agencies, this approach could also yield more accurate epidemiological data to estimate HIV incidence and enhance our ability to facilitate linkage to care. There are precedents in Europe and the U.S. for specimen self-collection with STI and HIV testing in public health laboratories, but they have not been scaled up on a widespread basis.
Second, from a public health perspective, I feel that false negatives are worse than false positives. Prior to the approval of the first dried blood spot test kit over 15 years ago, concern was expressed that people would become psychologically unstable (maybe even suicidal) if they received a positive result without a counselor present. Since such a kit is still on the market, I can only assume the risk of suicide must be pretty low – otherwise, FDA would have surely pulled it. Times have definitely changed since 1995, as well; HIV is no longer the inevitable “death sentence” it was before combination antiretroviral therapy became the standard of care, and the public has at least some consciousness of that fact. No, it’s the false negatives that are of greater concern, and the potential for people to use rapid tests as “point-of-sex” testing, or as permission to forgo condoms. The earliest that rapid tests can diagnose a new infection is around 20-30 days after infection, since they rely on antibodies to HIV that need time to crank up to detectable levels. If you hook up with a high-risk new partner who has seronegative acute HIV infection and is in that 20-30 day window before antibodies develop, his or her rapid test is going to be a false negative. We also know that during the window period, people tend to have very high viral loads and are therefore highly infectious to others. We’re going to have a tough time getting the message out that a negative result isn’t necessarily reassuring.
 Finally, there’s the issue of cost and what restrictions that will place on who’s using in-home rapid testing. Currently, to obtain a home specimen collection kit, you have to go to a pharmacy or order it online, at a cost of between $50 and $80. No price has been set yet for OraQuick, but it’s likely to be more than $20 and less than $60. Who’s going to be able to afford that kind of money in this economy? I feel like those using rapid tests at home are more likely going to be people with disposable income – often White, insured, and male – and may not be representative of those at greatest risk. Over the past six years I’ve been at UNC, most of my new patients have been young, Black MSM, generally working paycheck-to-paycheck. Given the CDC’s statistics showing this population experienced a 48% increase in incident HIV between 2006-2009, I doubt that those in greatest need of expanded testing are going to be able to avail themselves of this new option.
Finally, there’s the issue of cost and what restrictions that will place on who’s using in-home rapid testing. Currently, to obtain a home specimen collection kit, you have to go to a pharmacy or order it online, at a cost of between $50 and $80. No price has been set yet for OraQuick, but it’s likely to be more than $20 and less than $60. Who’s going to be able to afford that kind of money in this economy? I feel like those using rapid tests at home are more likely going to be people with disposable income – often White, insured, and male – and may not be representative of those at greatest risk. Over the past six years I’ve been at UNC, most of my new patients have been young, Black MSM, generally working paycheck-to-paycheck. Given the CDC’s statistics showing this population experienced a 48% increase in incident HIV between 2006-2009, I doubt that those in greatest need of expanded testing are going to be able to avail themselves of this new option.
What do you think? Will in-home HIV testing make a difference? Will that difference be good or bad? Talk with others about it, get additional perspectives, and decide for yourself. As members of the HIV treatment and advocacy communities, it’s up to us to start that dialogue and help our friends and loved ones make the best possible decisions about how to implement this new strategy in HIV testing.
Christopher Hurt, MD is a Clinical Assistant Professor in the Division of Infectious Diseases at the University of North Carolina at Chapel Hill School of Medicine. He graduated from the University of Florida College of Medicine in 2003, and went on to complete Brown University’s residency program in internal medicine at Rhode Island Hospital and The Miriam Hospital. He came to Chapel Hill in 2006 for his fellowship in infectious diseases at UNC and has been a faculty member since 2009.