Top 10 HIV Stories of 2016
by David Alain Wohl, MD
Professor, Division of Infectious Diseases, The University of North Carolina at Chapel Hill
Director, North Carolina AIDS Training and Education Center
Site Leader, The University of North Carolina Chapel Hill AIDS Clinical Research Site
1. Trump
2. Switch Frenzy
3. 2-Drug ART: Paddling Against the 3-Drug Current
4. Is HIV PrEP at a Tipping Point?
5. Start ART Now
6. Return of the Antibodies
7. Blip or Bloop: Is an unexpected low level HIV RNA level real?
8. DTG and the CNS
9. TAF in HBV
10. New HIV infections in US are down – a bit
Introduction
In typical years, a noteworthy development in the world of HIV would be the result of a landmark clinical trial or, perhaps a gem of a lab study with a novel finding. But, 2016 was not typical. What made the most difference for people living with HIV and their health care providers this past year was less a paper published or presented, than major shifts in our thinking about how best to prevent and manage HIV infection.
This was the year of the “shake up” and the eschewing of the good-enough status quo. HIV-positive patients and their providers, traditionally cautious in their approach to treatment, have more recently become willing to embrace change, including to antiretroviral regimens. Switch and Simplification have replaced Stay the Course as a guiding HIV therapeutics mantra. New drugs and formulations offer treatment options that are markedly easier and safer than the medications on hand just a year or two ago. Even the dogma regarding what constitutes highly active antiretroviral therapy is being questioned by daring ART minimalists for whom fewer drugs mean lower risks and costs. At the same time, the movement that advocated successfully for earlier administration of HIV therapy is now pushing for provision of medications the very same day the patient presents for testing. For all this radicalism there were also throwbacks to retro ideas, like using antibodies to fight HIV – an approach that was tried and failed early in the epidemic.
Overshadowing this evolution of thought were the results of the presidential election and the delivery of control of both the House and Senate, as well as a few governorships to the Republican Party. Perhaps no other development will have as much impact (think: comet, dinosaurs) on the future of people living with HIV than the 2016 elections – and that is where we start.
Trump
Even if you were celebrating in the wee hours of November 9 with a red baseball cap on your head, you would be hard pressed to defend the notion, given the available facts, that the outcomes of the 2016 election were good for people living with HIV infection. With funding for the Ryan White Care Act and its provisions supporting HIV care and medication already on the chopping block – despite being demonstrated to increase the lifespans of people living with HIV – history tells us that Republican control of the executive and legislative branches of government spells dark days ahead for this and other such public good works.
Our new president has made clear that a top priority during his first 100 days will be the destruction of the Affordable Care Act (ACA). This will not take an act of congress. Executive action alone can effective pull the plug on Obamacare. For instance, simply dropping the administration’s appeal of a federal judge’s ruling in House v. Burwell that the spending of funds to reimburse insurers for covering working-poor consumers was not appropriated by congress and, therefore unconstitutional, will deal a sufficiently fatal blow. Without this subsidy to insurers their costs will skyrocket, prompting them to pull out of the system, and to the implosion of the ACA.
Since it became law, the ACA has expanded health insurance coverage to 20 million people and it is estimated that less than 9% of all people in the US are now uninsured. People with and at risk for HIV infection have certainly benefited from the ACA. So, with its demise, there will be HIV-positive people left without coverage unless the new administration and congress provide a responsible alternative.
In addition to the killing of Obamacare, cuts in funding of Ryan White support for clinical care, case management, and medications would further erode the safety net for poor and working class people living with HIV. An ambitious plan to cut taxes, improve infrastructure, fortify borders, and replace the ACA with a new system of coverage will all cost money and it is reasonable to be concerned that social programs, like those that benefit the working poor with and at risk for HIV will be raided.
The Republican sweep can also impact HIV care and prevention through decisions made on NIH funding, or lack of it. In the past, funding for federal research has suffered when congressional control rested with the GOP and many of the most exciting HIV research initiatives such as developing and testing injectable antiretrovirals and microbicides for prevention, interventions to reduce HIV-associated inflammation, approaches to combat the opioid epidemic, and the HIV cure agenda, rely heavily on NIH funding.
The Bottom Line: Cumulatively, the very altered political reality we shall be living in will be much more significant to the person living with HIV than the buzzy issues described below. Debates about whether HIV replication can be suppressed with 2 versus 3 medications is a moot for the patient who cannot afford her asthma medicine, mammogram, or car ride to see her doctor. Predicting what a President Trump will do is a fool’s game. Instead, we will see what he and his administration do for the HIV-positive ‘little guy’.
“The only constant is change,” wrote Heraclitus 2,500 years ago. He also advised that “people must fight on behalf of the law as though for the city wall.” There will be many battles ahead for numerous noble causes and we, who care for people living with HIV, and those who claim to advocate for them will need to be vigilant, or else what we have built will crumble.
Switch Frenzy
Options breed choice and as safer, better tolerated, and more convenient antiretrovirals have become available they have tempted us to manipulate even effective HIV regimens. With a critical mass of such options at hand, we are increasingly discussing the merits of ‘simplification’ with our patients – trading in old, stodgy regimens for sleek new models. The current switch-mania it could be argued started in earnest with co-formulation of the pharmacological booster cobisistat with darunavir and atazanavir. This allowed us to gleefully abandon ritonavir and end a marriage that we had been seeking to leave for years. Even the diehards holding on to their beloved 4-pill a day lopinavir/ritonavir finally broke down and accepted a single tablet in their stead – their bowels have thanked them ever since. But it has been the availability of tenofovir alafenamide fumarate (TAF) in a slew of co-formulations that has really got the switch party hopping.
The replacement of tenofovir disoproxil fumarate (TDF) wherever it is found with TAF grants a wish high on an HIV provider’s list. A TDF-like drug with better renal and bone safety means we do not have to obsess about creatinine creep or t-scores. Taking up less physical space helps clinch the deal for lateral swap-outs of TAF for TDF, with only the looming specter for prior authorization to stand in the way. Switching, though, is not just about TAF. The single tablet of abacavir, lamivudine, dolutegravir has been useful for those of the guanosine analogue persuasion, and, a number of recent studies demonstrate more novel and radical modifications to successful regimens that can be accomplished, providing more convenience with less medicine.
Major recent studies of virologically suppressed patients whose ART was switched are listed in the table.
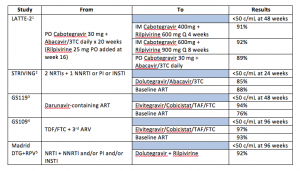
The Bottom Line: Messing with successful ART, once eschewed as playing cowboy/girl, is now required as part of good care. With improvements in ART, I look at each patient’s regimen to determine whether it is optimal or not. For example, those who initiated ART a decade ago may have been started on a boosted protease inhibitor regimen despite having no baseline drug resistance. It is unlikely most of these patients, were they to enter care today, would leave with a prescription for darunavir or atazanavir. As their age increases, so too does their risk of drug interactions and polypharmacy. Therefore, a discussion regarding regimen revamping is warranted. For others, longer term toxicity, like elevated lipids and osteopenia, or even persistent low-level intolerability that was just coped with can be addressed with a swap.
What switch studies also tell us is that maintaining suppression of HIV is easier than achieving an undetectable viral load and that even some forms of very minimal ART can keep a lid on HIV replication. This is nicely illustrated in the LATTE-2 trial where two agents with low to moderate barriers to drug resistance are able to maintain viral suppression following more standard induction therapy.
It is remarkable to stand back and reflect that for this once fatal and contagious viral infection, that took so many people, we now have treatment options that are more potent and safer than all our old handful-of-M&Ms-cocktails, and yet, no bigger than a Tic Tac or baby aspirin. Treatment will continue to advance, as described below. As it does we should be mindful of how far we have come and that we are still dealing with a virus that continues to hurt and kill.
1. Margolis DA, et al. AIDS 2016. Abstract THAB0206LB.
2. Lake J, et al. AIDS 2016. Abstract THAB0203
3. Huhn GD, et al. IDWeek 2015. Abstract 726.
4. DeJesus E, et al. ASM 2016. Boston MA. #087LB
5. Diaz A, Casado F, Dronda F, et al. Dolutegravir plus rilpivirine in suppressed heavily pretreated HIV-infected patients. Program and abstracts of the 21st International AIDS Conference (AIDS 2016); July 18-22, 2016; Durban, South Africa. Abstract TUPDB0106
2-Drug ART: Paddling Against the 3-Drug Current
It has been well over a year since the concept of using dolutegravir plus lamivudine as initial ART was introduced to the world and for some of us it still a tough idea to get used to. 6 Decades of indoctrination to hit hard with a 3 or 4 drug strategy has been difficult to shake. In addition, initial treatment with these two agents requires faith in the potency and resistance barrier of dolutegravir, which was slow at first but is growing.
The fate of this ART-lite strategy has been riding on a small study of 20 treatment-naïve patients in Argentina, the PADDLE study. Last year, the study team showed us remarkably brisk responses among those enrolled, with all participants achieving an undetectable viral load after 8 weeks of therapy.6
This summer week 48 data were presented and 18 of the 20 participants had maintained viral suppression.7 Of the two who were not suppressed, one was a suicide and the other, who had an HIV RNA level >100,000 copies/mL at screening, experienced virologic failure but re-suppressed on dolutegravir plus lamivudine without intensification or switch.
The Bottom Line: The PADDLE study is not quite the HIV therapeutics equivalent of landing a human on Mars. Rather, it’s more like the small rover that touched down on the planet and kicked up red dust. Gusty and going where no ART has gone before, PADDLE has pushed our boundaries and imagination. For sure, the features of dolutegravir are essential to this regimen’s simplicity, underscoring the need to consider not only antiretroviral quantity but also quality. Larger studies, of course, will need to confirm the PADDLE results before we embrace this and similar 2-drug regimens, and these are underway – but already what we have seen opens a brave new world where potent therapy could be cheaper and certainly safer than some of the big-guns we are deploying now.
6. Figueroa MI, et al. EACS 2015. Abstract 1066
7. Cahn P, et al. AIDS 2016. Abstract FRAB0104LB.
Is HIV PrEP at a Tipping Point?
TDF/FTC was approved for use as Pre-Exposure Prophylaxis (PrEP) to prevent HIV infection way back in 2012 – the year Mitt Romney challenged President Obama’s re-election. Although that may seem like a billion years ago, it wasn’t and since then the uptake of PrEP has been steadily increasing. As reported at the 2016 International AIDS Conference by the Centers for Disease Control and Prevention (CDC), about 80,000 people have been prescribed PrEP from 2012-2015.8 However, the CDC estimates there are 1.2 million people in the US whose risk for HIV makes them candidates for PrEP (including 490,000 men who have sex with men (MSM), 115,000 people who inject drugs, and 624,000 heterosexual men and women).9 Moreover, 76% of these PrEP users were men. Other data suggest that only a fraction of those prescribed PrEP are not white or male.10
Given disparities in access to medical care and HIV prevention messaging it may not be a surprising that white men were most likely to adopt PrEP. While PrEP uptake in this population is a good thing, those who can most benefit from PrEP are not receiving it. Recent modeling performed by the CDC estimates the lifetime risk of HIV infection to be 1 in 20 for African-American men, and 1 in 48 for African-American Women and Hispanic men. Bad, but it gets worse. MSM had an astounding 1 in 6 risk and this jumped to a mind boggling 1 in 2 for African-American MSM.11
The Bottom Line: PrEP is an effective and important tool in our shallow HIV prevention toolbox. Real-world studies have shown that it works. However, a number of studies show that there is incredibly limited awareness of the existence of PrEP among people who are candidates for it and among health care providers.
It should take nothing more than the risk faced by MSM, particularly MSM of color, to justify a massive push to get PrEP to all who can benefit from it, regardless of who they are, where they live, or how much they make. Certainly, PrEP in its current incarnation is not perfect. For now, it is still a pill that needs to be taken most every day. The TDF component can drop bone density a few percentage points, and PrEP does not protect against other major sexually transmitted infections (STI). Recent data show that among adolescent MSM, adherence to PrEP is pitiful.12 However, it still saves lives and money.
What do we need to do get PrEP from being a secret that has been best kept from those who are at risk and the primary care providers who care for them? As a provider who has become evangelical about PrEP, I believe what is needed to make this as accessible as oral contraceptives is exposure and action:
- Marketing: Few successful medications market themselves. The manufactures of TDF/FTC have belated realized this and are finally launching a full court marketing press including detailing of the drug to clinicians and direct-to-consumer advertising to potential users – especially in cities and regions where HIV incidence rates are high.
- Public Funding for non-drug PrEP Costs: Although the cost of TDF/FTC for PrEP is often covered by insurance or, for those who qualify, the manufacturer support program, many of the other costs associated with PrEP, such as clinic visits and STI testing, is not covered. This can be a tragic deal-breaker. Expanded access to PrEP needs to happen in places where people can get affordable health care including federally qualified health centers, community health centers, and health departments. An STI clinic that is not providing PrEP or at least offering a referral to a PrEP clinic is falling short of their mission.
- Don’t Ignore Injectors: PrEP works not just for prevention of acquisition of HIV via sex; injection drug users can also be protected. However, there is little attention paid to this group. If we do not want another Scott County, Indiana, PrEP education and provision needs to be folded into other efforts that target the opioid epidemic in rural America. Needle exchange and opioid substitution sites have to be added as venues where PrEP can be accessed.
- Primary Care Must Get on Board: It is not just the manufacturer of PrEP that needed to be convinced to jump-start efforts to get PrEP out there. Health care providers too must be accepting that their patients have sex, that sexual health is part of general health and thus should be discussed, and that PrEP is a biological intervention proven to prevent HIV and should be prescribed. By them. To their patients. The mindset among primary care regarding HIV prevention must change. Moralizing by providers about condoms, promiscuity, and gay sex will lead to people becoming infected and some dying. Primary care associations, medical, family practice, and OB/GYN residencies need to immediately include education and training about how to take a sexual history and about prescribing PrEP for those at risk. There are excellent resources for learning efficient techniques to ask about sexual health. The Ask, Screen, Intervene (ASI) training developed by the CDC, HRSA, NIH and the HIVMA provides excellent guidance (https://effectiveinterventions.cdc.gov/en/HighImpactPrevention/PublicHealthStrategies/AskScreenIntervene/ASIImplementationMaterials.aspx). Prescribing PrEP is about as complicated as writing for oral contraceptives and is a whole lot easier than treating Lyme Disease. So, to the primary care docs reading this who are not prescribing PrEP, please start doing so.
- Community Advocates Must Get on Board: Lastly, the same community advocates who advocate for fair drug pricing and defend studies like the START trial have to have skin in the PrEP game – and many do. These are the people that can best push to make PrEP better known and accepted by those at risk for HIV infection. Community voices must be heard by those who are reluctant to provide PrEP.
During the past year, an undercurrent of progress has been made. Stakeholders have started to mobilize to spread the word about PrEP. The first PrEP-related advertising has launched. My prediction is that PrEP will take off and the steep climb in uptake will be one of the best stories of 2017. Let’s all help to make it so.
8. Mera R, et al. AIDS Conference 2016; Durban, South Africa. Abstract TUAX0105LB
9. Smith D, et al. MMWR Morb Mortal Wkly Rep 2015;64:1-6
10. Bush S, et al. ASM Microbe / ICAAC 2016; Boston, MA. Abstract 2651
11. http://www.cdc.gov/nchhstp/newsroom/2016/croi-2016.html#Graphics
12. Hosek S, et al. AIDS 2016. Abstract TUAX0104LB.
Start ART Now
ART is for everyone regardless of CD4 cell count! No more T-cell thresholds! Starting ART early saves lives! You have heard it. I have heard it. We get it. Now that we do, new data suggests that we take the ‘earlier is better’ philosophy one step further and offer HIV medication with the first handshake. Two studies presented this year provide evidence for the feasibility of same day (or close to it) HIV testing and ART initiation. Both also report fairly astounding and, to some, counter intuitive, outcomes with this approach.
A team from San Francisco looked retrospectively at 39 patients, most with acute or recent HIV infection, who were offered ART at their first clinic visit rather than at a return visit following counseling and laboratory testing.13 Uptake of ART at the first visit was high at almost 95% and the time to virologic suppression was 1.8 months, half that of those who were offered ART under the more traditional multi-visit schedule.
A randomized trial (N=762) of same-day ART initiation versus traditional ART initiation after laboratory testing and counseling visits conducted in Haiti found that same-day ART led to greater uptake of ART (100% versus 92%), lower mortality (3% versus 7%), and higher suppression rates (54% versus 42%) – all differences were highly statistically significant.14
The Bottom Line: There are plenty of reasons to believe that time spent counseling and preparing a patient for a lifetime of HIV therapy is well spent. However, these studies suggest that there is a cost in many cases to delaying the start of ART, even for a few weeks. Loss to follow-up, time to suppression (and therefore, less infectiousness), and mortality in places like Haiti were all worse when ART was made a multi-step process. Same-day treatment of HIV, beyond providing rapid biological responses, also may work by sending a strong message that HIV is a serious infection that deserves immediate attention. Talk is good, but the ‘walk’ is found in medication and this is the hook that keeps many patients coming to clinic.
There are certainly logistical issues that clinics need to sort out, especially procuring a supply of medications – a mean feat given the insane patchwork of health care insurance coverage here in the US. In California ART is more accessible than in many other places in the county and the San Francisco clinic that reported their experience with rapid ART administration had a 5-day supply of medications available to dole out to those who needed it. In clinics where ART is readily available and for patients with easy immediate access to HIV medications, same-day ART should be explored as a way to improve retention in care and smooth those steps on the HIV care continuum cascade.
13. Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The Effect of Same-Day Observed Initiation of Antiretroviral Therapy on HIV Viral Load and Treatment Outcomes in a U.S. Public Health Setting. J Acquir Immune Defic Syndr. 2016 Jul 16. [Epub ahead of print]
14. Koenig S, Dorvil N, Severe P, et al. Same-day HIV testing and antiretroviral therapy initiation results in higher rates of treatment initiation and retention in care. JAIDS. 2016;19(suppl 5):64. Abstract WEAE0202.
Return of the Antibodies
It’s not just aviator sunglasses, Ghostbusters, and Brittney Spears that are back, once again we are looking at antibodies to fight HIV infection. The idea harks to the earliest days of the epidemic when anti-HIV immunoglobulin therapy was tried but proved to be an epic fail. We now understand that typical antibody responses to HIV are insufficiently neutralizing against the virus to be useful. However, a small proportion of those infected produce unusual antibodies that are broadly neutralizing and can target not only the virus present in that one person but even most other strains of the virus found in others. Importantly, such broadly neutralizing antibodies (bNAb) can be synthesized and are being studied to treat and prevent HIV infection.
Last year, one bNAb, VRC01, was reported to decrease plasma HIV RNA levels in viremic patients.15 In 2016, two NIH-supported studies were presented that showed infusions of VRC01 in patients whose suppressive ART was interrupted led to a more prolonged delay in viral rebound than expected based on data from other patients who had interrupted ART.16 Eventually, viral rebound did occur in those receiving the antibody and selection of virus that evaded the antibody was found. However, these studies provide the all-important proof-of-concept needed to light the fuse for future research initiatives. These include using bNAbs as adjuncts to ART, and as agents to help reduce the latent reservoir and therefore facilitate eradication..
One of the most exciting areas of BNAb research is as a preventive intervention like PrEP. The HIV Prevention Network Trial and HIV Vaccine Trials networks (NIH-funded) is conducting the AMP Study (http://ampstudy.org/about). In this trial 2,700 men and transgender women at risk for HIV acquisition are randomized to two different doses of VRC01 or to a placebo infusion to determine the safety and protective afforded against HIV of the BNAb.
The Bottom Line: Immunotherapy is dawning as an important tool in the treatment of malignancies and has become first line therapy for autoimmune conditions such as inflammatory bowel disease, psoriasis and rheumatoid arthritis. Infectious diseases such as hepatitis B and varicella have been treated with immunoglobulins as has, more recently, Ebola. Although many HIV providers may not yet be aware of bNAbs, it is likely they will be. Kinks need to be worked out, such as reducing production costs, and developing an array of antibodies to avoid viral resistance, but these are not insurmountable. So, if asked what is the next big thing in HIV, just say, “bNAbs”.
15. Lynch RM, Boritz E, Coates EE, et al Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 2015; 7:319ra206
16. Bar KJ, Sneller MC, Harrison LJ, et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med 2016 Nov 9 2016; 375:1807-1809
Blip or Bloop: Is an unexpected low level HIV RNA level real?
Jay has been my patient since I was an Infectious Disease fellow and I can’t forget the conversation we had back in 1992, soon after he was diagnosed during which I convinced him to start HIV therapy – with d4T and lamivudine (yikes!). A lot has happened since then, including some updates to his ART, and together we have grown older. His health has been excellent and his HIV RNA levels always undetectable, that is, until last month. A routine semi-annual viral load returned at 70 copies/mL (the lower limit of detection for the assay used is 20 copies/mL). Jay was in a panic. He has never missed a dose of his HIV medication and denied any new medications or supplements or recent vaccination. Was this just a blip? And, if so, what exactly caused it?
For years, we have dealt with occasional viral load blips as we have Facebook friend requests from beautiful people in Estonia – something that happens, which we don’t understand, and we don’t respond to. But, do blips really represent actual virus that have percolated above the rim of the limit of assay detection only to then simmer down? A small but provocative study conducted two experiments to answer this question.17
In the first phase, a standard WHO stock of HIV-1 was diluted to create 50 samples of blood with viral loads of 142, 71, 36, 18, and 9 copies/mL. Each batch of 50 samples were then shipped to three different commercial laboratories for analysis using the Cobas AmpliPrep/Cobas TaqMan v2.0 HIV-1 assay (Roche). As expected, there was some variability in quantification, but for the standards with 36 copies/mL and 18 copies/mL of HIV-1 RNA, that is close to but below the assay’s limit of detection, 66% and 18% of the tests, respectively, yielded results with values of ≥50 copies/mL. That is, even though the specimens truly contained less than 50 copies/mL of virus, a substantial proportion run through the test returned with a result above this level. None of the lowest concentrations of virus (9 and 4 copies/mL) were ever reported as detectable.
In the second part of the study, a single plasma specimen from four patients with prior suppression and who now had a value that indicated low level virus (range 50-93 copies/mL) was run repeatedly on the Cobas TaqMan. Retesting of the same sample with the newly detectable HIV RNA result produced a repeat result of
The Bottom Line: At present, when a treated HIV-positive patient’s viral load unexpectedly becomes detectable, a call is made to a now nervous patient, a viral load and maybe resistance test is ordered, and blood is redrawn. Based on the data from this study, the investigators propose a new paradigm with less hassle and cost – simply having the same sample with the unexpected blip saved and tested again. According to their data, in the vast majority of cases where the patient has been adherent (i.e., pre-test probability of true rebound is low), the repeat result will be undetectable and all can breathe a sigh of relief. If not, then the call can be made and repeat testing conducted. It is notable that new FDA guidance for clinical research trials allow for confirmation of virologic outcomes using retest of the last collected specimen.
Understanding that such blips are most likely a function of the inherent variability of an assay, can save patients anxiety and the health care system money. For this to work, though, commercial laboratories need to be willing to save specimens for a couple weeks or so, at least from those with blips, and a mechanism for ordering reflex same-specimen testing created. Both of which seem very do-able.
17. Eron J, Garner W, Wei L, et al. Retesting of suspected low-level HIV-1 viral load blips: A new paradigm to prevent extra clinic visits and unnecessary patient anxiety. Abstract 948. IDWeek 2016
Doultegravir and the CNS
As described above, the integrase inhibitor dolutegarvir has proved to be a potent and durable antiretroviral agent. Initial reports of a high barrier to resistance, well beyond that seen with raltegravir and elvitegravir, have allowed it to be used where before only a boosted protease inhibitor would do. This year, however, there were rumblings, especially in Europe, that dolutegravir might also stand out as by having some unsavory neuropsychiatric effects.
- Germany:18 The rates of integrase inhibitor discontinuations due to adverse effects among over 1,700 patients treated with this class of antiretrovirals at two large medical centers were 9.4% for elvitegravir, 6.8% for dolutegravir and 4.1% for raltegravir. The majority of discontinuations due to neuropsychiatric events was highest for dolutegravir: 5.6% for dolutegravir, 0.7% for elvitegravir, and 1.9% for raltegravir (n=678). Being female, older than 60 years of age, and coadministration with abacavir, were each associated with dolutegravir neuropsychiatric adverse events. In a handful of patients rechallenged with dolutegravir, CNS symptoms returned.
- Spain:19 In 1,091 patients at a Barcelona clinic initiating or switching to ART that included an integrase inhibitor, discontinuations over the next year were not statistically significantly different among the three agents of this class: raltegravir, elvitegravir, and dolytegarvair. However, fewer patients discontinued raltegravir due to adverse events (28%) compared to patients on elvitegravir (62%) or dolutegravir (31%) (P = 0.0083). Yet, when looking at central nervous system (CNS) problems speficially, significantly more patients receiving dolutegravir (88%) than raltegravir (35%) or elvitegravir (19%) discontinued their treatment for these CNS adverse effects (P = 0.0046). Interestingly, older age was again found to be an independent of early discontinuation of integrase inhibitor therapy.
- Netherlands:20 Among 556 dolutegravir treated patients, 15% stopped treatment (14% of the 556 for adverse effects). A host of neuropsychiatric adverse effects including insomnia and other sleep disturbances, depression, anxiety and psychosis, led the reasons for dolutegravir discontinuations and as in the German study, the risk seemed to be greater when abacavir was also part of the regimen.
Retrospective cohort studies have their flaws and should be interpreted with caution. Reporting and channeling bias can over-call events and the lack of standardization in the collection of data can miss important findings. In an initial response to concerns of CNS effects the makers of dolutegravir conducted an analysis using adverse event reporting received during four industry-sponsored clinical trials that enrolled patients who were treatment-naïve and could be randomized to dolutegravir (SPRING-2, FLAMINGO, SINGLE, ARIA).21 Specifically, rates of psychiatric events including insomnia, nightmares/abnormal dreams, anxiety, depression, and suicidality among those receiving this integrase inhibitor were compared across the various study arms. Together these studies enrolled 2,634 participants, 1,315 of whom received dolutegravir. In general, the rates of each symptom were similar among those treated with dolutegravir and the comparator regimens. Interestingly, in the double-blind SINGLE trial, where the control arm included efavirenz, rates of most all the psychiatric events (except for suicidality, which was very low in both arms), were much higher than those reported in the other studies included in the analysis. The investigators surmise that the inclusion of efavirenz and the double-blind design of SINGLE may explain this difference. However, it is also very plausible that the more careful assessments for neuropsychiatric events in this trial could also have revealed an issue that would be missed otherwise.
The Bottom Line: Dolutegravir is an important and useful antiretroviral that is generally well-tolerated. The recent reports of neuropsychiatric adverse events with integrase inhibitors, and dolutegravir in particular, may be a signal of an off-target effect of these medications. Given their key role in modern day HIV therapy, this potential problem needs to be addressed head-on. Additional research to better understand if this is real and if it is why it happens and who is at greatest risk is necessary, particularly as we increasingly use this and other drugs of this class in older patients, who these preliminary studies indicate may be at heightened risk.
18. Sabranski M et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. Glasgow 2016. Oral abstract O124
19. Padilla M, Rojas J, Gonzalez-Cordon A, et al. Tolerability of integrase inhibitors in a real-life setting. 18th International Workshop on Comorbidities and Adverse Drug Reactions in HIV, September 12-13, 2016, New York.
20. de Boer M et al. Intolerance of dolutegravir containing cART regimens in real life clinical practice. AIDS 2016. Published online: 24 September 2016. dos: 10.1097/QAD.0000000000001279. http://journals.lww.com/aidsonline/Abstract/publishahead/Intolerance_of_dolutegravir_containing_cART.97671.aspx
21. Quercia R et al. Psychiatric adverse events from the DTG ART-naive phase 3 clinical trials. Glasgow 2016. Poster abstract P210.
TAF for HBV
It is fortuitous for people living with both HIV and HBV infections that TDF and FTC are effective against both viruses. Trouble can brew though when for one reason or another, like a change in renal function, the TDF needs to be stopped. Until this year, for co-infected patients jettisoning TDF meant adding on the anti-HBV agent entecavir to the new TDF-less ART regimen. It is no surprise then that when TAF was first shown to be effective against HIV, many wanted to know whether it would also be as good as TDF for HBV.
The answer is, yes. In two trials in HBV mono-infected patients, one for HBeAg-negative (N=425) and the other for HBeAg-positive patients (N=873), participants were randomized to TAF 25 mg versus TDF 300 mg daily.22, 23 At 48 weeks, there was no significant difference between the two treatments in the proportion with a plasma HBV DNA level
A smaller single arm study enrolled 72 patients with HIV and HBV co-infection who were all switched to the single tablet elvitegravir/cobicistat/FTC/TAF.24 At enrollment 96% were on TDF and 86% had an undetectable HBV DNA level. At 48 weeks, 92% had an undetectable HBV DNA level and HIV suppression was maintained, while renal and bone indices improved.
The Bottom Line: Evidence that TAF is as effective as TDF for treating HBV is welcome news to those infected with both of these viruses. While in the US HIV-HBV co-infection is not common, it is in many other regions, including Asia and Africa. These data are, therefore, comforting to the few patients in our practice who have suppressed HIV and HBV and now or later need to switch from TDF. The larger impact will be where HBV is endemic and for these people, TAF’s demonstrated ability to treat HBV is a big deal.
22. Buti et al. A Phase 3 study of tenofovir alafenamide compared with tenofovir disoproxil fumarate in patients with HBeAg-negative, chronic hepatitis B: week 48 efficacy and safety results. International Liver Congress, Barcelona, abstract GS06, 2016.
23. Chan HL et al. A Phase 3 study of tenofovir alafenamide compared with tenofovir disoproxil fumarate in patients with HBeAg- positive chronic HBV: week 48 efficacy and safety results. International Liver Congress, Barcelona, abstract GS12, 2016.
24. Gallant J, Brunetta J, Crofoot G, et al. Brief Report: Efficacy and Safety of Switching to a Single-Tablet Regimen of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide in HIV-1/Hepatitis B-Coinfected Adults. J Acquir Immune Defic Syndr. 2016 Nov 1;73(3):294-298
New HIV infections in US are down – a bit
For many years we have been told that the annual number of new diagnoses of HIV infection in the US has remained fixed at around 50,000 people. And, that 50K stat has hung around the necks of all who are dedicated to HIV prevention, weighing on them and sometimes forcing back-to-the-drawing-board planning to beat down the stubborn incidence of HIV. This year the CDC released new figures that indicate a 19% drop in new HIV diagnoses from 2005 to 2014.25 Moreover, for women, the decline was 40% with the greatest drop among African-American women (42%).
However, before we all start popping corks in celebration there was also some sober news from the report. HIV diagnoses actually increased among MSM, especially those who are Latino or African-American (Asian-American and American Indian/Alaska Native MSM also saw large upticks in new HIV diagnoses but the absolute number of cases in these groups was relatively small). Younger MSM of all races and ethnicities drove the increases reported. Mildly reassuring was the finding that over the most recent years the rate of increase in HIV detection among African-American MSM appeared to be slowing, including among younger men in this category.
The CDC report also looked at geographic disparities in the epidemic. It is a pitiful picture. Southern states account for 44% of all people living with HIV, despite accounting for about 37% of the US population. Further, the death rate among HIV-positive people in the US South is three times that of people living in other states. In general, the deeper the South you go, the worse the death rate for HIV-infected people. Same regional trend, not surprisingly, goes for the proportion of infected people unaware of their diagnosis.
The Bottom Line: That 10,000 fewer people are now being diagnosed with HIV is super-awesome and is likely due to a combination of the earlier initiation of ART thus preventing transmission, more forgiving ART to keep people suppressed, and some uptake in PrEP. The news is welcome and means that something seems to be finally working.
Of course, that something is not working equally for everyone and the data are clear that, once again, it is the US South that is lagging. It is difficult to know where to begin when reacting to yet another map that has the southern states shaded with badness. The almost same pattern can be found in maps that indicate incarceration rates, male-female imbalance, voting rights denial for those with criminal records, lack of Medicaid expansion – and, of course – maps that show which states vote blue and which vote red. The road to further reducing new diagnoses of HIV infection runs through Dixie and if this region’s now emboldened leadership resists expanded HIV testing, the provision of ART to those in need, and the funding of care and services for people living with HIV, there it will meet a very dead end.
25. http://www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-data-trends-fact-sheet-508.pdf