A research team led by Damaris Lorenzo, PhD reports in Nature Genetics the discovery of the genetic and molecular basis for a neurological syndrome caused by variants in the SPTBN1 gene. SPTBN1 instructs neurons and other cell types how to make βII-spectrin, a protein with multiple functions in the nervous system. Children carrying these variants can suffer from speech and motor delays, as well as intellectual disability. Some patients have received additional diagnosis, such as autism spectrum disorder, ADHD, and epilepsy. Identification of the genetic variants that cause this broad spectrum of disabilities is the first important milestone to finding treatments for this syndrome. Dr. Lorenzo is an assistant professor of Cell Biology and Physiology and member of the Neuroscience Center and the Carolina Institute for Developmental Disabilities at the UNC School of Medicine.
Lorenzo first learned about patients with complex neurodevelopmental presentations carrying SPTBN1 variants from Dr. Queenie Tan, MD, PhD, a medical geneticist, and Becky Spillmann, MS, CGC, a genetic counselor, both members of the NIH-funded Undiagnosed Disease Network (UDN) site at Duke University and authors in the study. They connected with Margot Cousin, PhD, a geneticist associated with the UDN site at the Mayo Clinic and co-first author in the study, who had also collected clinical information from SPTBN1 variant carriers. Other clinical genetics teams learned about their efforts and joined the study. The cohort of individuals affected by SPTBN1 variants continues to grow. Lorenzo and colleagues have been contacted about new cases after they published a preprint of their initial findings last summer. “Identifying the genetic cause of rare diseases such as the SPTBN1 syndrome requires pooling knowledge from several patients to establish common clinical and biological patterns. Fortunately, the advent of affordable gene sequencing together with the creation of databases and networks that facilitate the sharing of information among clinicians and investigators has vastly accelerated the diagnosis of rare diseases”, added Lorenzo. “To put our case in historical perspective, βII-spectrin was co-discovered 40 years ago through pioneering work that involved my UNC colleagues Keith Burridge and Richard Cheney, as well as my postdoctoral mentor Vann Bennett. However, its association with disease eluded us until now”, Lorenzo explained.
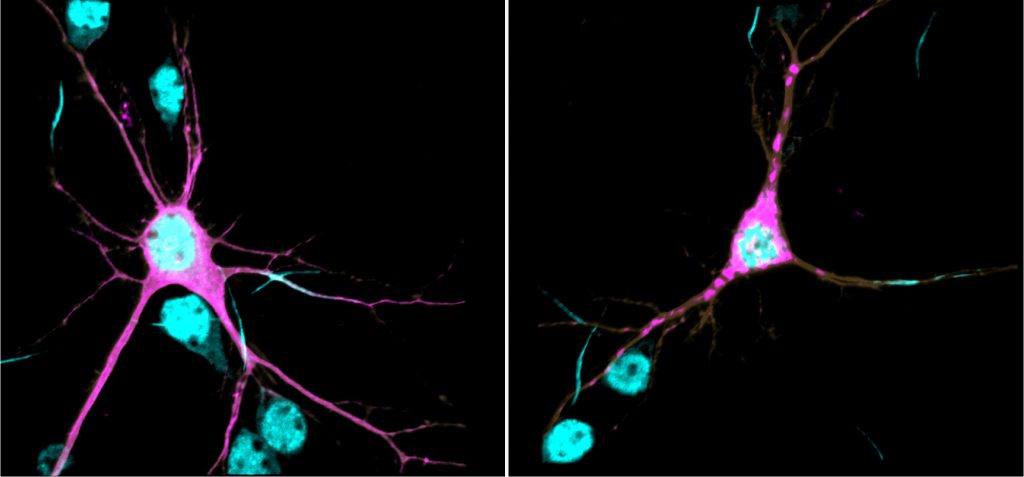
βII-spectrin is tightly associated with the neuronal cytoskeleton and forms an extended scaffolding network that provides mechanical integrity to membranes and correctly positions molecular complexes throughout the neuron. In previous research published in PNAS in 2019, Lorenzo had shown that βII-spectrin is essential for normal brain wiring in mice and for proper transport of organelles and vesicles in axons, a process that enables normal development, maintenance, and function of neurons. In this new study, Lorenzo’s research team showed that at the biochemical level the genetic variants identified in the patients are sufficient to cause protein aggregation, aberrant association of βII-spectrin with the cytoskeleton, impair axonal organelle transport and growth, and change the morphology of neurons. These deficiencies can permanently alter how neurons connect and communicate with each other, which is thought to underlie the etiology of neurodevelopmental disorders. The team showed that reduction of βII-spectrin levels only in neurons disrupts structural connectivity between cortical areas, a deficit also observed in brain MRI of some patients. In collaboration with Sheryl Moy, PhD, professor of Psychiatry and director of the Mouse Behavioral Phenotyping (MBP) Core at UNC, they found that these mice have developmental and behavioral deficits consistent with presentations observed in the patients.

“Now that we have established the methods to assign likelihood of pathogenicity to SPTBN1 variants, our immediate goal is to gain new molecular and cellular insights into their mechanisms of action and evaluate strategies with potential clinical interventions”, said Lorenzo. To this end, her team will use neurons differentiated from patient-derived induced pluripotent stem cells in collaboration with Adriana Beltran, PhD, an assistant professor of Genetics and director of the UNC Human Pluripotent Cell Core (hHPSC) and will continue to tap into molecular modeling predictions in collaboration with Brenda Temple, PhD, professor of Biochemistry and director of the UNC Structural Bioinformatics Core, both authors on the paper. “As a basic science investigator, it is very satisfying when you can use your knowledge and tools to provide answers to patients”, Lorenzo said. “I first witnessed this thrill of scientific discovery as a graduate student 15 years ago when my PhD lab identified the genetic cause of the first spectrinopathy and it has been a powerful professional motivator since”, said Lorenzo, referring to the discovery of variants in a different spectrin gene that cause spinocebellar ataxia type 5, led by her then mentor, Laura Ranum, PhD. “Besides the immediate relevance to the affected patients, insights from the SPTNB1 syndrome will inform discovery in other complex disorders with overlapping pathologies”, concluded Lorenzo.
Link to paper in Nature Genetics
Along with Lorenzo, other members of the Lorenzo lab are co-authors in the Nature Genetics paper. They include co-first author Blake Creighton, lab research technician in the Lorenzo lab; Reggie Edwards, Lorenzo lab graduate student; Keith Breau, Lorenzo lab graduate student at the time of this research; Deepa Ajit, a postdoctoral fellow in the Lorenzo lab; Sruthi Dontu, Simone Afriyie, and Julie Bay, all undergraduate members of the Lorenzo lab; and Liset Falcon, lab research technician in the Lorenzo lab at the time of this research. Other UNC-Chapel Hill collaborators and co-authors in the paper include Kathryn Harper, Ph.D., project manager in the MBP Core; and Lorena Munoz and Alvaro Beltran, both research associates in the hHPSC. This research in the Lorenzo lab was funded by grants from the National Institutes of Health and the National Ataxia Foundation.
News courtesy of Mark Derewicz at UNC Health Communications
