Normal Motor Neurons
Contents
- A. Isolated Motor Neuron Cell Bodies
- 1. Why isolate spinal motor neuron cell bodies?
- 2. How can one isolate highly purified adult motor neurons?
- 3. What criteria identify the isolated cells as motor neurons?
- 4. How intact are the isolated cell bodies?
- 5. How can one label newly synthesized proteins in isolated motor neuron cell bodies?
- 6. How can one measure RNA synthesis in isolated motor neuron cell bodies?
- 7. How can one measure the volume of an isolated motor neuron cell body?
- B. Normal Motor Neurons: Biochemical Characterization
- 1. How much DNA does the nucleus of a motor neuron contain?
- 2. How much RNA do motor neuron cell bodies contain?
- 3. How much protein do motor neuron cell bodies contain?
- 4. What are the major protein constituents of motor neuron cell bodies?
- 5. How stable are motor neuron cell body proteins post mortem?
- 6. How different are the proteins in the motor neuron cell body and the ventral gray matter?
- 7. How different are the proteins in the motor neuron cell body and motor axon?
- 8. How different are the proteins in spinal motor and sensory axons?
- 9. What fraction of its protein content does the motor neuron cell body export each day?
- 10. How much new protein travels by fast transport to the motor axon each day?
- 11. How much acetylcholinesterase enters the motor axon each day?
- 12. How much choline acetyltransferase and acetylcholinesterase do lumbar motor neuron cell bodies contain?
- 13. What fraction of the acetyltransferase and acetylcholinesterase activity in the ventral gray matter is found in motor neuron cell bodies and their proximal dendrites?
- 14. How early during development can choline acetyltransferase and acetylcholinesterase be detected in motor neurons?
- C. Normal Motor Neuron Size: Morphological and Biochemical Relationships
- 1. Why focus upon motor neuron size?
- 2. How are nucleolar, nuclear and cell body size related in motor neurons?
- 3. Is the cytoskeleton responsible for maintaining these size relationships?
- 4. What are the mechanical properties of the cytoskeleton in motor neuron cell bodies?
- 5. What are the solubility properties of the cytoskeleton in motor neuron cell bodies?
- 6. What are the major proteins in the cell body cytoskeleton?
- 7. Does the cytoskeleton cage lipofuscin granules?
- 8. Do large motor neurons contain more nuclear DNA than small motor neurons?
- 9. Do large motor neurons synthesize more RNA than small motor neurons?
- 10. Do large motor neurons synthesize more protein than small motor neurons?
- 11. Does growth hormone play a role in motor neuron size?
- 12. Does growth hormone help to maintain motor neuron size in adult animals?
My colleagues and I focused much of our attention upon the structure and function of normal spinal motor neurons. In doing so, we have generated information about these cells that can be used not only to understand normal motor neurons more completely, but also as a basis for comparing them to injured and diseased motor neurons. I begin with a summary of the central method used in this research – the isolated motor neuron cell body preparation.
A. Isolated Motor Neuron Cell Bodies
1. Why isolate spinal motor neuron cell bodies?
Despite more than a century of study, there is still much to learn about the chemistry of adult spinal motor neurons. A major reason for the deficiency in our understanding of motor neurons is that it is difficult to perform comprehensive, quantitative analyses of their constituents without interference from neighboring cells. It is possible to select and study specific constituents in adult motor neuron cell bodies in situ by methods such as immunohistochemistry. However, by freeing motor neuron cell bodies from surrounding tissue, one can use several different methods to display and analyze a wide range of their constituents, without guessing at which specific molecules might be present. Isolated motor neuron cell bodies are especially amenable to quantitative analyses, and we wanted to be able to detect quantitative differences among motor neurons. Advances in the sensitivity of analytical techniques make it increasingly easy to quantify the constituents of individual or groups of motor neuron cell bodies.
All the available methods for studying the chemistry and structure of motor neurons have both advantages and disadvantages. No one method can best answer every question. Methods for isolating motor neuron cell bodies are readily learned, easily carried out without unusual equipment or technical skills, even when manipulating single cells, and have a number of advantages. In addition to their being uncontaminated by other cells, isolated motor neurons have the advantage that one can use comprehensive methods of analysis, such as 2-D gel electrophoresis or mass spectrometry, to separate and identify individual protein constituents in normal, injured and diseased motor neurons. Knowledge of the total amounts of cell constituents was also necessary in many of our studies and isolated cell bodies, such as those in which we measured the total amount of DNA, RNA and protein in normal cell bodies or quantified changes in newly synthesized protein and RNA after injury to the motor axon. In studies of the structure of the motor neuron cell body and proximal dendrites, isolated cell bodies permit morphometric measurement of the entire cell body volume, unaffected by problems encountered with analyses in situ with fixation or incomplete inclusion of the cell body in tissue sections. In contrast to tissue culture methods that require young motor neurons, one can study cell bodies isolated from adult spinal tissue with fewer concerns about how accurately the cells reflect features of the mature motor neuron.
On the other hand, there are disadvantages associated with isolated cell body preparations. The motor neuron plasma membrane is disrupted when cell bodies are dissociated from the surrounding tissue, due to avulsion of postsynaptic membrane along with synaptic terminals during isolation. This excludes portions of the plasma membrane from study and increases membrane permeability, necessitating special methods to prevent the loss of soluble constituents from the cell. As with all methods, the introduction of other artifactual changes in the cells by the isolation method is always a risk. Moreover, the axon is usually sheared from the cell body during isolation, limiting one’s ability to study cell body-proximal axon interactions. Dissociation of the motor neuron from the ventral gray matter not only limits one’s ability to study their relationship to surrounding cells, but also eliminates the spatial cues that help make their identity so obvious. It is not difficult, however, to identify and select many motor neurons during the isolation procedure, because of their unusually large size and responses to injury of the ventral root.
Our studies on isolated motor neuron cell bodies have convinced us that much can be reliably learned, and sometimes best learned, about these cells by first isolating them.
2. How can one isolate highly purified adult motor neurons?
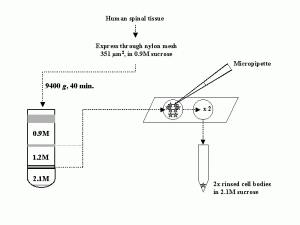
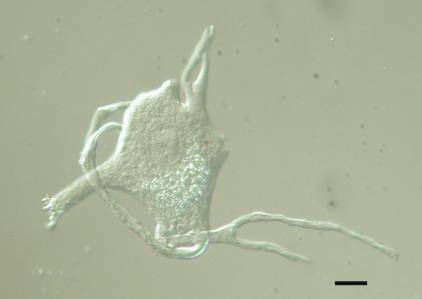
Our laboratory developed two methods for isolating motor neuron cell bodies. The first utilizes nylon sieving screens of known pore size to eliminate much of the contaminating tissue and was used primarily to isolated bovine cell bodies (Capps-Covey and McIlwain, 1975; Weil et al., 1977; Weil and McIlwain, 1981). The second method involves picking up individual dissociated cell bodies and washing them free of contaminants and has been used by us to isolate motor neurons from human, cow, mouse, frog and rat spinal tissue ![]() . The second is the preferred method, because it yields less contaminated cell bodies and is more easily applied to small animals that have fewer spinal motor neurons. It is easy to learn, requires no special equipment other than a low speed centrifuge and inverted light microscope equipped with a micromanipulator, and permits one to collect hundreds of cell bodies in one day.
. The second is the preferred method, because it yields less contaminated cell bodies and is more easily applied to small animals that have fewer spinal motor neurons. It is easy to learn, requires no special equipment other than a low speed centrifuge and inverted light microscope equipped with a micromanipulator, and permits one to collect hundreds of cell bodies in one day.
3. What criteria identify the isolated cells as motor neurons?
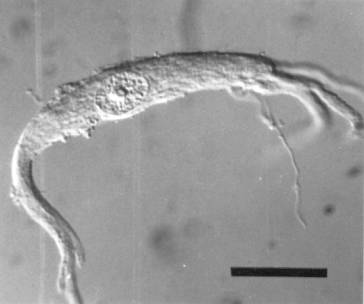
Little experience is needed with our isolation methods to realize how easily one can identify the cell bodies of spinal motor neurons. In our preparations, there is nothing else that looks like them. Motor neurons have large, often euchromatic nuclei with a single nucleolus, multipolar cell bodies that are easily distinguished from non-neurons and from smaller interneurons. Bovine ![]() and human
and human ![]() spinal motor neurons usually have a stellate appearance, with their primary dendrites emerging from several points around the circumference of a rounded cell body. Those isolated from the grass frog
spinal motor neurons usually have a stellate appearance, with their primary dendrites emerging from several points around the circumference of a rounded cell body. Those isolated from the grass frog ![]() often have dendrites emerging from opposite ends of a triangular or fusiform cell body. The cell bodies of most alpha motor neurons are much larger than those of gamma motor neurons, although in the frog, gamma motor neurons are believed to be absent in the lumbar spinal cord (Gray, 1957). In addition, cell bodies isolated by our methods have, as expected, high concentrations of the cholinergic enzymes, choline acetyltransferase and acetylcholinesterase (Weil et al., 1977). After transection of the ventral root, the isolated motor neurons exhibit the expected increases in size, total protein and acetylcholinesterase (Sinicropi et al., 1982). Even the smallest cell bodies we isolate send their axons into the ventral root, since axotomy increases their size in the same way it alters the size of larger motor neurons (McIlwain and Hoke, 1999).
often have dendrites emerging from opposite ends of a triangular or fusiform cell body. The cell bodies of most alpha motor neurons are much larger than those of gamma motor neurons, although in the frog, gamma motor neurons are believed to be absent in the lumbar spinal cord (Gray, 1957). In addition, cell bodies isolated by our methods have, as expected, high concentrations of the cholinergic enzymes, choline acetyltransferase and acetylcholinesterase (Weil et al., 1977). After transection of the ventral root, the isolated motor neurons exhibit the expected increases in size, total protein and acetylcholinesterase (Sinicropi et al., 1982). Even the smallest cell bodies we isolate send their axons into the ventral root, since axotomy increases their size in the same way it alters the size of larger motor neurons (McIlwain and Hoke, 1999).
4. How intact are the isolated cell bodies?
Early investigations by others into the properties of neurons isolated from mature animals indicated that, in addition to the loss of the axon and the distal dendrites, damage also occurred to the plasma membrane of the cell body. We documented this damage in isolated bovine spinal motor neurons by electron microscopy (Hester et al., 1978), showing evidence of avulsion of postsynaptic membrane on the cell body that increased its permeability by Procion Yellow, a fluorescent dye that is normally excluded by the plasma membrane. Later, we found that cryoprotection of the spinal tissue prior to isolation of motor neuron cell bodies improved the light microscopic appearance of the isolated cells, especially those isolated from the grass frog (Sinicropi et al., 1989), leaving them with longer dendrites and smoother surfaces, as well as increasing the number of cells recovered.
The increased permeability of the isolated spinal motor neurons implied the possible loss of soluble cell constituents during the isolation procedure. We confirmed the loss of soluble proteins during the isolation of bovine lumbar motor neurons (Weil et al., 1977). Following a protocol for reversibly decreasing the solubility of the soluble enzyme choline acetyltransferase (Fonnum, 1968), we used low ionic strength, low pH media to increase the retention of that enzyme 30-fold, but also to increase the soluble protein content of the isolated cell bodies 7-fold (Weil et al., 1977). The later use of cryoprotection further increased the total protein content of the isolated cell bodies and reduced the loss of newly synthesized proteins from them (Sinicropi et al., 1989).
Using these precautions to minimize the loss of soluble constituents from cell bodies during their isolation, we have been able to obtain a large body of information about the structure and function of motor neurons that is reproducible and useful. When we have addressed questions that were also investigated by other methods of analysis, our findings on isolated cell bodies have been consistent with data obtained by those methods.
5. How can one label newly synthesized proteins in isolated motor neuron cell bodies?
For studies on newly synthesized protein, we first explored a number of different methods for labeling the cells with radioactive amino acids. We quickly determined that, like previous attempts by other investigators, direct labeling of isolated motor neuron cell bodies gave on average only a few cpm of labeled protein in each cell body. Since the measurement of radioactivity could be made for many, pooled cell bodies over long period of time, this level of labeling was acceptable for some experiments. We found, however, that one can achieve over two orders of magnitude higher labeling in frog motor neurons by exposing the spinal cord to the radioactive precursor first and then isolating the labeled cell bodies. This procedure allows one to examine the fate of newly synthesized proteins in motor neurons, but it cannot distinguish between changes in the rate of synthesis, degradation or export of new protein from the cell body.
A description of the conditions needed for efficient radiolabeling of spinal motor neurons from frog has been published (McIlwain and Hoke, 1994). In that study, approximately the same results were obtained using 3H-leucine or 35S-methionine. We do not know whether the more efficient labeling of motor neurons in situ is a consequence of damage to the protein synthetic machinery during cell isolation or a result of the disruption of the cellular environment in which the neuron exists in the spinal cord. Preliminary attempts to apply the procedure to rodents and to post mortem human tissue have been unsuccessful.
Optimal labeling of motor neuron protein also depends upon whether or not the spinal tissue is frozen before cell body isolation. We have reported that cryoprotection of frog spinal cord after radiolabeling protein but prior to freezing the sample can increase by ten-fold the recovery of newly synthesized protein in isolated motor neuron cell bodies, relative to frozen, but unprotected tissue (Sinicropi et al., 1989). Lower, but still useful levels of labeled protein are recovered in cell bodies isolated from unfrozen spinal tissue that is not cryoprotected.
6. How can one measure RNA synthesis in isolated motor neuron cell bodies?
In contrast to the procedure for labeling protein, satisfactory levels of RNA labeling can be achieved using isolated motor neuron cell bodies. To ensure that RNA synthesis per se is being measured, we used a nuclear run-on technique to trap labeled, elongating RNA chains in the nucleus, taking advantage of the permeability of isolated cell bodies to deliver labeled and unlabeled precursor nucleotides to the nucleus. The procedure we used for isolated motor neurons has been published (Sato et al., 1994; Burgess and McIlwain, 1994).
7. How can one measure the volume of an isolated motor neuron cell body?
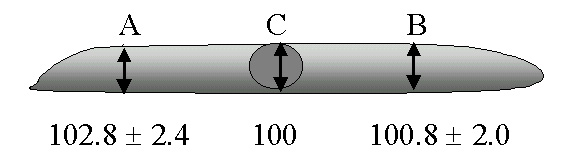
The area and volume of an isolated motor neuron cell body can be reproducibly quantified using an inverted light microscope, preferably equipped with interference contrast optics. Most of the isolated cell bodies we have examined, including those from the frog, are flat, nearly cylindroidal structures. The circumference of the cell body can be judged and outlined reproducibly and conveniently by making a digital image of the cell and using a standard program for computer-assisted morphometry. For volume measurements, we routinely measured the height (z axis) in the middle and at each end of the cell body, using the microscope’s graduated focus controls, multiplying the average height times the averaged area to obtain the volume of a wafer-like cylinder. When suspended in 1.2M sucrose solutions and sandwiched between two No.1 glass coverslips, cell body height varies little across the cell. For example, in a series of four experiments on a total of 120 normal frog lumbar motor neuron cell bodies, the height on each end of the cell varied less than 3% from the height in the middle of the cell ![]() . With no top coverslip, normal frog cell bodies are slightly more ellipsoidal, with the height at the two ends of the cell body being 87.9% ± 18 or about 13% less than the height at the middle (n=40 cells). Addition of the top coverslip increases the cell body area by about 50%.
. With no top coverslip, normal frog cell bodies are slightly more ellipsoidal, with the height at the two ends of the cell body being 87.9% ± 18 or about 13% less than the height at the middle (n=40 cells). Addition of the top coverslip increases the cell body area by about 50%.
B. Normal Motor Neurons: Biochemical Characterization
1. How much DNA does the nucleus of a motor neuron contain?
In both the plant and animal kingdoms, a positive correlation exists between the size of a cell and its DNA content. Since spinal motor neurons are among the largest mononucleate cells in animals (striated muscle cells are multinucleate), they had been suspected to have more than the normal diploid amounts of DNA found in somatic cells. We investigated this possibility in two different studies (McIlwain and Capps-Covey, 1976; Sato et al., 1994) both of which used flow cytometry and staining of DNA with propidium iodide to examine the DNA content in lumbar motor neurons. The first study used nuclei purified from isolated bovine spinal motor neurons (McIlwain and Capps-Covey, 1976), while the second study (Sato et al., 1994) used nuclei obtained from isolated frog spinal motor neurons. In both studies, we found only diploid amounts of DNA in motor neuron. Direct measurement of DNA in bovine nuclei indicated that they contain an average of 5.9 picograms/nucleus.
2. How much RNA do motor neuron cell bodies contain?
Spinal motor neurons organize their ribosomes into large blocks called Nissl bodies, the RNA of which stains intensely with methylene blue. Most of the RNA in motor neuron cell bodies is ribosomal RNA. In the course of characterizing isolated bovine motor neurons, we found an average RNA content of 1353 ± 37.2 picograms of RNA per cell body (Capps-Covey and McIlwain, 1975). In later, unpublished studies on human lumbar spinal motor neurons, we utilized a more sensitive luciferase-based assay for RNA and found an average of 955.3 ± 37.2 pg RNA/ cell body in isolated human motor neuron cell bodies. Using the rule of thumb that there are roughly equal amounts of ribosomal RNA and ribosomal protein in cells, we predict that the cell bodies of human lumbar motor neurons contain about 1000pg (1ng) of ribosomal protein.
3. How much protein do motor neuron cell bodies contain?
Quantitative analyses of motor neuron proteins have been central to many of our studies. From the beginning, for example, we wanted to know the total protein content of the isolated motor neuron cell bodies we studied. While there is justifiably strong emphasis on studying individual proteins in motor neurons, some of the questions we asked required information about the total protein content of isolated cell bodies.
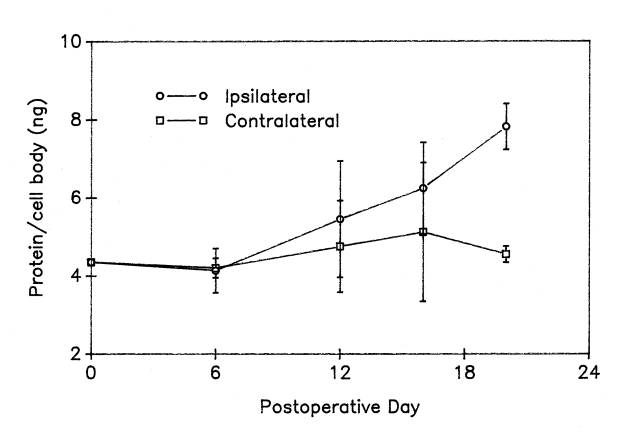
As one would expect, motor neurons of different sizes contain different amounts of protein. After refining our methods for preserving soluble protein within motor neurons during the isolation procedure (see above), we found that cell bodies isolated from the lumbar spinal cord of cows contain an average of 20.5 ± 3.4 nanograms/cell; from human beings, 13.1 ± 1.6 ng/cell; from the grass frog (R. pipiens), 4.35 ± 0.87 and from the rat, 4.22 ± 0.41 ng/cell. Species differences in protein content undoubtedly reflect differences in cell body size, but lacking a method to measure total protein within individual motor neuron cell bodies, this size relationship was not quantified in single cells. Estimates of total cell body protein content were used in a number of different ways: e.g., to determine the number of cell bodies needed for gel electrophoresis; to answer questions about the export of protein from the cell body ![]() ; to determine the amount of protein remaining in the cell body cytoskeleton of extracted motor neurons
; to determine the amount of protein remaining in the cell body cytoskeleton of extracted motor neurons ![]() ; and to examine cell body responses to injury
; and to examine cell body responses to injury  . Knowing the total cell body protein content, one can also estimate the fraction of the total represented by individual proteins or groups of proteins. For example, the predicted content of ribosomal protein in human motor neuron cell bodies (1ng) would represent 1ng/13.1ng or 7.6% of the total protein in human cell bodies.
. Knowing the total cell body protein content, one can also estimate the fraction of the total represented by individual proteins or groups of proteins. For example, the predicted content of ribosomal protein in human motor neuron cell bodies (1ng) would represent 1ng/13.1ng or 7.6% of the total protein in human cell bodies.
4. What are the major protein constituents of motor neuron cell bodies?
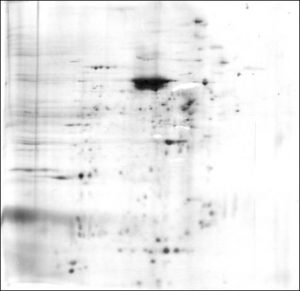
A thorough knowledge of any cell would include the identity and function of every protein contained in that cell. We are currently a long way from such an understanding of motor neurons. New proteomic technologies using mass spectrometry to identify large numbers of proteins at high levels of sensitivity now offer hope for major advances in our understanding of motor neuron proteins. These technologies were unfortunately not available during the period of most of our research. However, the advent of two-dimensional polyacrylamide gel electrophoresis (2-D PAGE) in the early 1970s did present new opportunities to separate, display and explore many major proteins within spinal motor neurons.
2-D PAGE can separate and detect (but alone cannot not easily identify) thousands of proteins, given sufficient amounts of starting material for analysis. For isolated spinal motor neuron cell bodies, that number is usually less than 500 total proteins, because of limits in the number and protein content of cell bodies that can be isolated and studied in experiments lasting a few days. Only a few of those proteins were identified in our electrophoretic studies. There are also limits to the kinds of proteins present on 2-D gels. Proteins present in very low amounts within cells can escape detection on the gels. Proteins must also be soluble in the reagents used in 2-D PAGE, and motor neurons contain substantial amounts of protein that may not meet this criterion ![]() . Special conditions must also be employed to display proteins that are very basic (i.e., that have high isoelectric points) or have very high or very low molecular weights.
. Special conditions must also be employed to display proteins that are very basic (i.e., that have high isoelectric points) or have very high or very low molecular weights.
img these limitations, 2-D PAGE has proven to be quite useful in exploring the most abundant individual proteins in motor neurons. We have used 2-D PAGE to examine the proteins of motor neuron cell bodies isolated from bovine (Weil and McIlwain, 1981; Brock and McIlwain, 1985), human ![]() and frog spinal cord (Sinicropi and McIlwain, 1983). We have detected hundreds of silver-stained protein spots on these gels. Using co-migration with standard proteins, we have identified several cytoskeletal proteins: alpha and beta tubulin, the low, middle and high molecular weight neurofilament proteins, and actin (Sinicropi and McIlwain, 1983). The relative amounts of most of these proteins have been estimated for cell bodies isolated from frog (Sinicropi and McIlwain, 1983) and bovine tissue (Brock and McIlwain, 1985). By performing mass spectrometry on motor neuron cell bodies isolated from species whose genome has been sequenced, it should now be possible to identify many of the remaining proteins in motor neuron cell bodies .
and frog spinal cord (Sinicropi and McIlwain, 1983). We have detected hundreds of silver-stained protein spots on these gels. Using co-migration with standard proteins, we have identified several cytoskeletal proteins: alpha and beta tubulin, the low, middle and high molecular weight neurofilament proteins, and actin (Sinicropi and McIlwain, 1983). The relative amounts of most of these proteins have been estimated for cell bodies isolated from frog (Sinicropi and McIlwain, 1983) and bovine tissue (Brock and McIlwain, 1985). By performing mass spectrometry on motor neuron cell bodies isolated from species whose genome has been sequenced, it should now be possible to identify many of the remaining proteins in motor neuron cell bodies .
5. How stable are motor neuron cell body proteins post mortem?
While the delay between the time of death and the time of tissue preparation can be kept short when studying experimental animals, such as the grass frog, it can be 0.5-1 hour after death before bovine spinal cord can be placed on ice and many hours before human spinal tissue is chilled and prepared for experiments. Thus, we wished to ascertain how stable the proteins that appear on 2-D gels of spinal tissues might be post mortem. In a series of experiments on bovine spinal tissue (Brock and McIlwain, 1985), we compared the 2-D protein patterns of ventral gray matter (VGM), spinal roots and isolated motor neuron cell bodies from bovine lumbar spinal tissue left a room temperature for 24h to tissue placed on ice and prepared for experiments within 1h.
The results demonstrated a striking stability in many proteins from VGM, spinal roots and motor neuron cell bodies exposed to room temperature for 24h (Brock and McIlwain, 1985). On the gels of VGM protein we found evidence of breakdown of the major astrocytic protein, glial fibrillary acidic protein (GFAP). Breakdown of that protein had begun by at least 1h after death, if not during the life of the animal, and progressed during the 24h period at room temperature. There was also an indication of some decrease in actin, tubulin and neurofilament protein in both ventral roots and motor neuron cell bodies isolated from spinal tissue after the 24h period, although statistically insignificant in all instances except for tubulin in the ventral roots. We concluded that the proteins appearing on the gels provide a reasonably accurate representation of the in vivo state of those proteins.
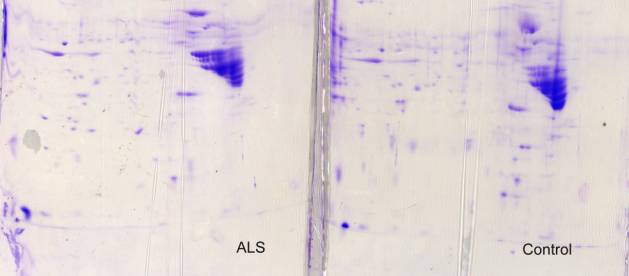
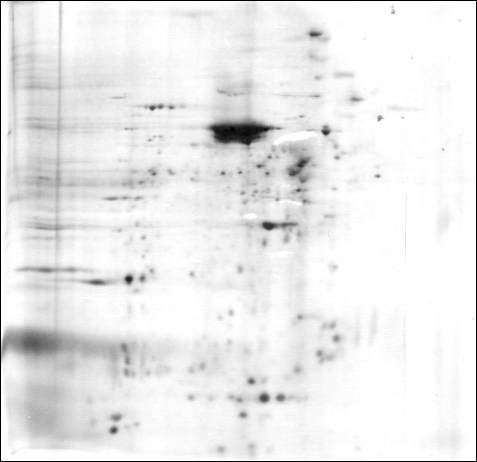
Two-dimensional gels of normal human VGM, especially those stained with Coomassie blue ![]() , contained a tremendous predominance of GFAP breakdown products, far exceeding that seen in bovine VGM after 24h at room temperature. This may relate to the longer post mortem delay, albeit mostly at 4 °C, for human tissue, to the longer human lifetime and possible increase in the number of astrocytes, or to a difference in the stability of human and bovine GFAP.
, contained a tremendous predominance of GFAP breakdown products, far exceeding that seen in bovine VGM after 24h at room temperature. This may relate to the longer post mortem delay, albeit mostly at 4 °C, for human tissue, to the longer human lifetime and possible increase in the number of astrocytes, or to a difference in the stability of human and bovine GFAP.
6. How different are the proteins in the motor neuron cell body and the ventral gray matter?
Motor neurons probably contain less than one-quarter of the total protein in the ventral gray matter. This rough estimate is based upon our calculation that approximately 2.8 million motor neurons are present in 50g (wet weight) of bovine lumbar ventral spinal cord trimmed of some of its white matter. If 50g of ventral spinal cord contains about 2.5g of protein and an isolated motor neuron cell body contains about 20.5ng protein, then all motor neuron cell bodies in that amount of spinal tissue contain a total of about 57mg of protein or 2.3% of the total protein in the ventral spinal cord trimmed of much of its white matter. Motor neuron dendrites are estimated to have about ten times the volume of the cell body, indicating that less than 25% of the trimmed ventral spinal cord protein is in motor neuron cell bodies and dendrites. Thus, if motor neuron proteins are substantially different from the proteins in the neuropil around them, one would predict that there should be large differences in the protein composition of motor neurons and the entire ventral gray matter.
Using 2-D PAGE to test this possibility, we first examined the gel patterns of bovine lumbar spinal proteins that were soluble in 50mM phosphate buffer (pH 7.4). The same amount of protein (40 mg) was analyzed in each sample. We found many differences in the soluble proteins in isolated motor neuron cell bodies and the ventral gray matter from which they were obtained (Weil and McIlwain, 1981). Some differences were quantitative, while others appeared to be qualitative. As would be expected of motor neurons isolated from ventral gray matter, all the major motor neuronal proteins were observed on gels of proteins extracted from ventral gray matter. Some of them were highly concentrated in motor neurons, compared to ventral gray matter. Conversely, ventral gray matter contained proteins not seen on gels from motor neurons. Twenty of the 50 most prominent spots in the VGM did not match to the 50 most prominent spots in the cell bodies.
In a later study (Brock and McIlwain, 1985) using a stronger extraction method and the more sensitive silver staining procedure, we again compared the protein composition of bovine lumbar and cervical ventral gray matter and isolated motor neurons. We extracted the isolated cell bodies and spinal tissue with O’Farrell’s lysis buffer, containing 2% SDS, 9.5M urea, 2% NP-40 and 4mM dithiothreitol. Again, there were many differences in the 2-D gel patterns of proteins from ventral gray matter and isolated motor neuron cell bodies, with most cell body proteins visible on the VGM gels, and many VGM proteins not seen on the cell body gels. Slight contamination of the isolated motor neuron cell bodies by GFAP may have occurred, although this possibility was not rigorously tested.
It was difficult to determine how many more proteins appeared on the gels in these experiments, relative to the first series of experiments on readily soluble proteins, since different stains were used in the first (Coomassie blue) and second (silver) experiments. Nonetheless, it was clear that many of the same cell body and VGM proteins appeared on gels in both experiments. However, there was some evidence that more new spots appeared on the cell body gels in the second vs. the first series of experiments than was the case for the VGM gels in the two experiments, suggesting the presence of more relatively insoluble proteins in the isolated cell bodies.
The results from the bovine studies illustrate the necessity of separating the motor neurons from the surrounding neuropil, if one wishes to study proteins specifically contained in motoneurons. They also provide a means by which one can compare changes in motor neuron proteins with changes in the surrounding tissue, since changes also occur both in motor neurons and in the surrounding tissue in ALS and after motor axonal injury.
7. How different are the proteins in the motor neuron cell body and motor axon?
The cell body is the site of synthesis of all proteins in motor neurons except those proteins that might enter the cell from the outside (e.g., by endocytosis, viral infection, etc.). Different proteins can be preferentially exported to the axon or dendrites, causing their distribution to be non-uniform within the cell. The axons of spinal motor neurons leave the spinal cord through the ventral roots (VR) and most of the proteins derived from the VR are likely associated with motor axons. The latter assertion is based upon morphometric analyses of bovine VR (Weil and McIlwain, 1981) and frog VR (Sinicropi and McIlwain, 1983). Between one-third and 40% of the cross-sectional area of the lumbar ventral roots is occupied by the axoplasm of motor neurons and proteins and about the same fraction is occupied by myelin. Since myelin contains comparatively little protein, it contributes few proteins to the 2-D gel patterns. The remaining cellular constituents of the ventral root (unmyelinated axons, Schwann cells, fibroblasts, capillaries) represent less than 20% of the cross-sectional area of the ventral roots. Albumin from blood vessels constitutes the most abundant protein from the latter fraction of the ventral root. Thus, most of the proteins on electrophoretic gels of the ventral roots probably belong to motor axons.
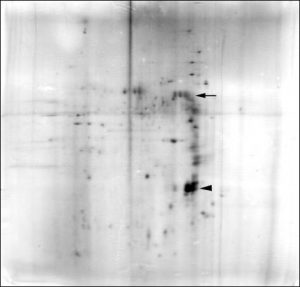
We sought evidence for differences in the proteins within motor neuron cell bodies and motor axons by applying 2-D PAGE first to bovine tissue. Substantial qualitative and quantitative differences were found in the cow in the soluble protein fractions obtained from VR and motor neuron cell bodies isolated from the lumbar spinal cord (Weil and McIlwain, 1981). When membrane-bound proteins were included in later studies on bovine (Brock and McIlwain, 1985), frog (Sinicropi and McIlwain, 1983) and human tissue ![]() , we again found many differences in the protein patterns of VR and isolated cell bodies.
, we again found many differences in the protein patterns of VR and isolated cell bodies.
Some cytoskeletal proteins were matched and quantified between frog VR and isolated cell bodies (Sinicropi and McIlwain, 1983). These quantitative data were useful in experiments on injured frog motor neurons, where axotomy-induced changes in specific cytoskeletal proteins differed in the axon and cell body. The observation that tubulin and neurofilament proteins increased in the cell body, but decreased in the remaining axon after transection of the ventral root suggested the possibility that those proteins were transiently being “dammed up” within the injured cell body (Sinicropi and McIlwain,1983). This possibility was strengthened by data obtained in the final study of our laboratory (McIlwain and Hoke, 2005) ![]() .
.
8. How different are the proteins in spinal motor and sensory axons?
There is a striking similarity in the protein composition of ventral (motor) and dorsal (sensory) spinal roots in cow (Weil and McIlwain, 1981) and in human beings ![]() . 2-D PAGE of the ventral and dorsal roots were qualitatively identical in these species, but were not analyzed for quantitative differences. This similarity could be useful in future compositional analyses of motor and sensory nerves. For us, electrophoretic analyses of ventral and dorsal roots proved very useful in a separate study on sporadic ALS, where abnormalities appeared in about one-half of the cases analyzed (Brock and McIlwain, 1984). The abnormalities reflected the presence of displaced astrocytes in the nerve roots, as described in more detail below
. 2-D PAGE of the ventral and dorsal roots were qualitatively identical in these species, but were not analyzed for quantitative differences. This similarity could be useful in future compositional analyses of motor and sensory nerves. For us, electrophoretic analyses of ventral and dorsal roots proved very useful in a separate study on sporadic ALS, where abnormalities appeared in about one-half of the cases analyzed (Brock and McIlwain, 1984). The abnormalities reflected the presence of displaced astrocytes in the nerve roots, as described in more detail below ![]() .
.
9. What fraction of its protein content does the motor neuron cell body export each day?
This question has been raised for many years and is still unsettled. Weiss (1967) estimated that primary sensory neurons may export to their axon roughly 10% of their newly synthesized protein each day. Despite substantial effort, we were unable to answer this question for either total protein or newly synthesized protein in spinal motor neurons.
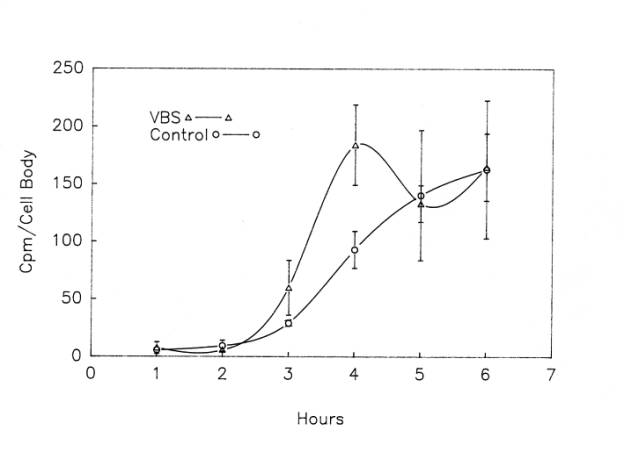
In our first effort to determine the amount of total protein leaving the motor neuron cell body by axonal and dendritic transport and possible release to the extracellular space, we exposed frog lumbar spinal cord to vinblastine sulfate (VBS), a microtubule inhibitor that blocks most export processes. VBS, rather than colchicine, was used, because VBS had been reported to block export more completely than colchicine (Komiya and Kurokawa, 1980) and because we had preliminary evidence that 25 mM colchicine inhibited in situ protein labeling in motor neurons by about 46%. We then compared the total cell body protein in those motor neurons to those isolated from spinal cord not exposed to VBS. Two methods were used to quantify the total protein in isolated cell bodies. The first method, a micro Lowry assay, indicated that 21h incubation at 17°C in the presence of 100 uM VBS caused a 25.8% increase in the total protein. However, a second method, using colloidal gold, showed no increase in cell body protein in the presence of 50 uM or 100 uM VBS during a 5h or 21h incubation period at 17°C. In addition to assay differences, doubts about whether VBS might partially inhibit protein synthesis confounded these experiments, as explained below. Although we found no evidence for the export of large amounts of total protein, we viewed our results as inconclusive.
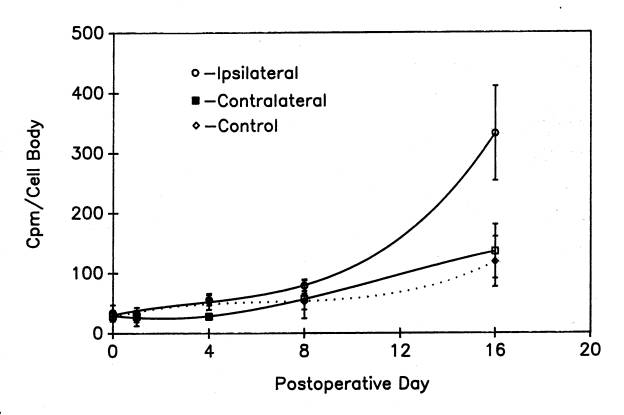
We then attempted to measure the export of newly synthesized (radiolabeled) protein from frog motor neuron cell bodies. Two experimental approaches were explored, one using VBS and the other using a pulse-chase paradigm. Neither approach gave unequivocal results. Based upon our first series of experiments with 100 uM VBS, we reported (McIlwain and Hoke, 1988) that approximately one-half of the new protein synthesized by frog motor neurons leaves the cell body during a 4h labeling period.
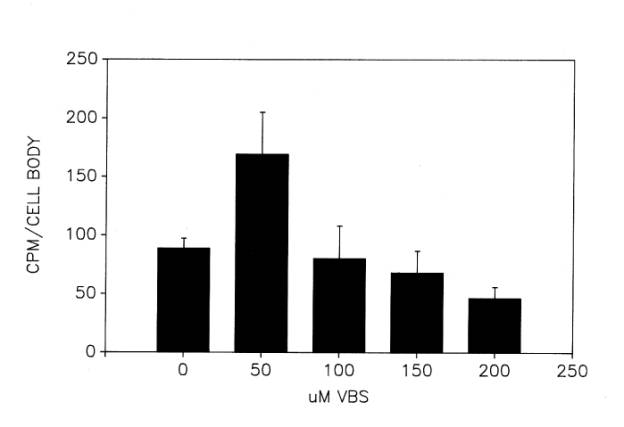
As we began to try to reproduce this result, we encountered several problems. First, despite some assurance from the literature that VBS does not inhibit protein synthesis, we were still uncertain as to whether our initial results were obtained under conditions in which VBS affected only export and that its effect was maximal. In a series of unpublished experiments, we investigated more extensively the optimal concentration of VBS ![]() and time of incubation
and time of incubation ![]() for inhibiting export. In the presence of increasing concentrations of VBS between 0-200 uM, radiolabeling of motor neuron cell bodies in hemisected lumbar spinal cord peaked at 50 uM VBS. Concentrations above 50 uM VBS appeared to suppress labeling progressively. As for the time course of labeling, 50 uM VBS caused the largest elevation in cell body protein labeling at 3 or 4h of incubation at 17°C, the stimulatory effect diminishing inexplicably by 5 and 6h of incubation
for inhibiting export. In the presence of increasing concentrations of VBS between 0-200 uM, radiolabeling of motor neuron cell bodies in hemisected lumbar spinal cord peaked at 50 uM VBS. Concentrations above 50 uM VBS appeared to suppress labeling progressively. As for the time course of labeling, 50 uM VBS caused the largest elevation in cell body protein labeling at 3 or 4h of incubation at 17°C, the stimulatory effect diminishing inexplicably by 5 and 6h of incubation ![]() . Based upon these results, which indicated narrow windows of efficacy in the use of VBS, we used 4h incubation periods and 50 uM VBS in subsequent experiments, described later in this section.
. Based upon these results, which indicated narrow windows of efficacy in the use of VBS, we used 4h incubation periods and 50 uM VBS in subsequent experiments, described later in this section.
A second area of concern was substantial variation in radiolabeling motor neuron protein. Over the course of the experiments with VBS, we determined four sources of this variation: 1) When the tissue was not cryoprotected in ethylene glycol for 90 min after the labeling period (Sinicropi et al., 1989), more than 90% of the labeled protein was lost from cell bodies isolated from spinal tissue. 2) cell-to-cell variation was clearly related to motor neuron size (McIlwain and Hoke, 1994); 3) cord-to-cord labeling was also variable, possibly related to differences in motor neuron size in different animals (McIlwain and Hoke, 1994); and 4) large seasonal variations occurred in labeling, with the highest levels of labeling obtain during the summer and autumn (McIlwain and Hoke, 1994).
We attempted to compensate for these sources of variation by cryoprotecting the labeled tissue for 90min before cell isolation, determining the number of spinal cords and isolated cell bodies required to minimize variations in mean labeling values, and by using only experiments performed in the summer and autumn of four consecutive years for our final analyses. Moreover, in 2 of 4 sets of experiments, we controlled for animal-to-animal variation by using opposite sides of the spinal cord from the same animals for control and VBS-treated cell body isolations. Despite our attempts to maximize VBS action and minimize variability in radiolabeling, the results of four additional studies using VBS were inconclusive ![]() . Variation in cell body protein labeling contributed significantly to the inconsistent findings. In the first two of these four studies, we found nearly 50% of the newly synthesized protein was exported from motor neuron cell bodies, just as we had reported (McIlwain and Hoke, 1988) before introducing the above controls. However, in the two later studies, 50 uM VBS inhibited the total labeled protein in motor neuron cell bodies, rather than increasing it by blocking its export
. Variation in cell body protein labeling contributed significantly to the inconsistent findings. In the first two of these four studies, we found nearly 50% of the newly synthesized protein was exported from motor neuron cell bodies, just as we had reported (McIlwain and Hoke, 1988) before introducing the above controls. However, in the two later studies, 50 uM VBS inhibited the total labeled protein in motor neuron cell bodies, rather than increasing it by blocking its export ![]() . We were never able to determine the reason for this reversal in the VBS effect. Thus, three of five sets of experiments, including the initial published study (McIlwain and Hoke, 1988), indicated that about one-half of the new protein leaves motor neuron cell bodies in 4h. The remaining two series of experiments showed inhibition of protein labeling by 50 uM VBS.
. We were never able to determine the reason for this reversal in the VBS effect. Thus, three of five sets of experiments, including the initial published study (McIlwain and Hoke, 1988), indicated that about one-half of the new protein leaves motor neuron cell bodies in 4h. The remaining two series of experiments showed inhibition of protein labeling by 50 uM VBS.
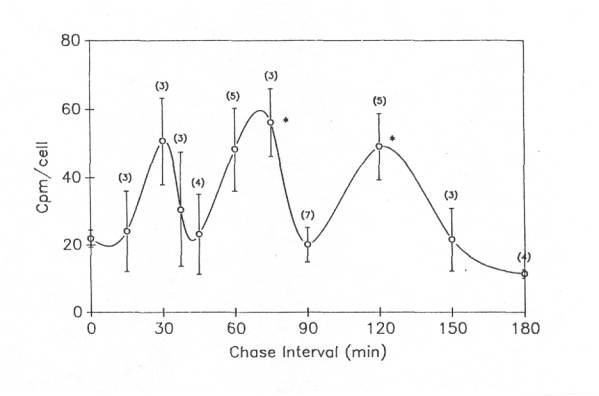
The second experimental approach we took to this question utilized a pulse-chase paradigm, in which proteins in frog motor neuron cell bodies were first radiolabeled with 3H-leucine, and then the label was “chased” with non-radioactive leucine (5mM) to inhibit further incorporation of 3H-leucine. One side of the spinal cord was used for a 2h pulse to label the motor neurons and the other side, also labeled during that 2h period, was then “chased” with cold leucine for periods of 15 -180 min. Three to seven different experiments were performed at each time point. Unexpectedly, three peaks were found when the chase interval was plotted against the mean cpm/cell body after the pulse period (0 min.) for all experiments ![]() . The middle peak was the largest, having about 3 times as much label per cell as found at the end of the pulse period. The cpm/cell body returned to the level of the pulse period (i.e., the level at 0 min.) after the first two peaks and fell below the pulse period after the third peak. Despite large variations in cell body labeling among different experiments, the second and third peaks during the chase period were significantly (p<0.05) higher than cpm/cell at the end of the pulse period. When radioactivity in cell bodies isolated after the chase interval in each individual experiment was plotted as a percent of the radioactivity per cell after the pulse period, rather than as cpm/cell body, and the results then averaged for all experiments, a similar multiphasic curve was observed. However, the relative peak amplitudes differed, with the peaks at 37.5min, 75min and 150min during the chase period being 630%, 580% and 1145% of the pulse period values.
. The middle peak was the largest, having about 3 times as much label per cell as found at the end of the pulse period. The cpm/cell body returned to the level of the pulse period (i.e., the level at 0 min.) after the first two peaks and fell below the pulse period after the third peak. Despite large variations in cell body labeling among different experiments, the second and third peaks during the chase period were significantly (p<0.05) higher than cpm/cell at the end of the pulse period. When radioactivity in cell bodies isolated after the chase interval in each individual experiment was plotted as a percent of the radioactivity per cell after the pulse period, rather than as cpm/cell body, and the results then averaged for all experiments, a similar multiphasic curve was observed. However, the relative peak amplitudes differed, with the peaks at 37.5min, 75min and 150min during the chase period being 630%, 580% and 1145% of the pulse period values.
If there is truly a waxing and waning of labeled protein in motor neuron cell bodies after the 2h labeling period, then there are at least two possible explanations for them. First, as much as two-thirds of the new protein may enter the axon and/or dendrites from the cell body during a 2h labeling period and the transported protein may then return to the cell body in waves several times during the chase period. Second, there may be periodic hydrolysis of labeled protein within the cell body, with retention and reincorporation of the label that is not inhibited by the presence of 5mM non-radioactive leucine. In order for this to be so, free, labeled amino acids would be retained in vivo, but lost during the isolation of motor neuron cell bodies. This is possible, since the cell bodies are isolated under conditions that retain proteins, but probably not free amino acids and the radioactivity measured is that which is precipitable with TCA. Thus, the disappearance of much of the label during the repeated low points in the multiphasic pattern may represent the artifactual loss of free, labeled amino acids during the isolation procedure, whereas during the radioactive peaks, more of the isotope is in labeled proteins that are retained during isolation and precipitated by TCA.
To test the possibility that reincorporation of label occurs during the chase period, we performed a series of four experiments in which two inhibitors of protein synthesis (250 mg/ml of puromycin and 100 mg/ml of cyclohexamide) were added to the chase medium of lumbar hemicords from one side of 6 frogs, in addition to the usual 5mM non-radioactive leucine. Following a 2h pulse and 75 min. (peak 2) chase period, cell bodies were isolated from the tissue and their radioactive protein content was compared to cell bodies isolated from lumbar hemicords of the opposite side of the frogs, which were chased in the absence of the protein inhibitors. In these four experiments, the average cpm/cell body protein from hemicords chased in the presence of puromycin and cyclohexamide was nearly the same (112.8 ± 87.9%) as in cell bodies isolated from hemicords chased in the absence of the inhibitors, indicating little or no additional protein labeling during the chase period. However, there was significant variation in the results of individual experiments: in two of the four experiments labeling was less in the presence of the inhibitors than in their absence; in the other two experiments, it was higher than in controls. In addition, incorporation rates of these experiments, performed in November – February, were low and variable. While the experiments were inconclusive, we suspect that little protein synthesis occurs during the chase period, given the presence of two inhibitors of protein synthesis, as well as 5mM ”cold” leucine. Thus, we favor the first hypothesis; namely, that new protein repeatedly leaves the cell body by a fast transport mechanism and returns to it rapidly – perhaps from the dendrites, which are short enough to permit such “reverberation” from their termini over the 3h period of these experiments.
The upshot of these two laborious projects was that one-half to two-thirds of new protein from motor neuron cell bodies may be exported within 4 hours. However, our inconsistent results with VBS and peculiar findings in pulse-chase experiments rendered this interpretation inconclusive. Ultimately, the expense of these unpublished experiments, both in time and funds, led to their termination.
10. How much new protein travels by fast transport to the motor axon each day?
The experiments in the previous section did not distinguish among export of new protein to the axon, the dendrites or by exocytosis to the outside of the cell body. We attempted to estimate the fraction of new protein exported daily to the axon by fast transport (McIlwain and Hoke, 1988) by comparing the labeled protein in isolated frog motor neuron cell bodies with labeled protein within the ventral roots. Using a concentric incubation chamber in which axonal labeling via transport could be distinguished from labeling via the extracellular space, we found that only a small fraction (0.5-12%) of the newly synthesized protein appeared in the motor axon by fast transport during the 4h labeling period. We also found evidence that the exported protein did not mix with all the cell body protein before leaving it, indicating that some new proteins are preferentially exported from the cell body. An attempt to block fast axonal transport using monensin was unsuccessful, because this agent showed a dose-dependent inhibition of protein labeling in the range of 0-500nM monensin. In successive experiments, monensin occasionally showed an elevation in cell body protein labeling, but inhibition occurred in two-thirds of the 36 samples tested. These experiments were performed without cryoprotection of the labeled cell bodies during isolation, and any resulting loss of labeled protein from the cell body would decrease the estimate of the fraction of labeled protein exported to the axon by fast transport. These results also did not address or preclude the possibility that labeled protein had entered the axon and then returned to the cell body.
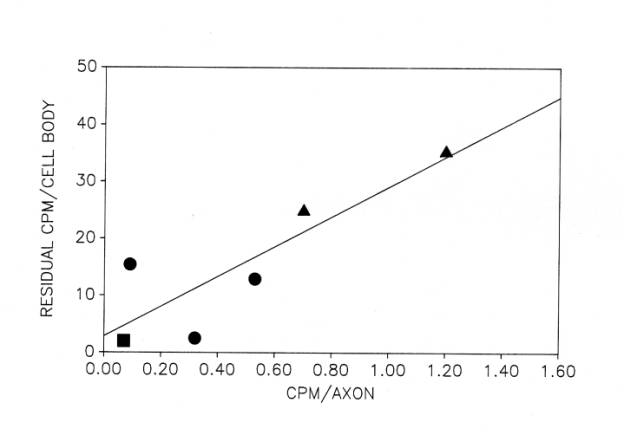
The small fraction of total new protein that is rapidly transported into the axon may be related to the 10% of total labeled protein that remains in motor neuron cell bodies that are isolated from frozen, non-cryoprotected tissue ![]() . It is possible that the new protein released by freezing and thawing is not membrane-bound. Conversely, the residual new protein may be membrane-bound protein, some of which is rapidly transported into the motor axon during the period of protein labeling. The basis for this suggestion is our finding of a strong correlation between the residual new protein in cell bodies isolated from frozen, unprotected tissue and the new protein detected in their axons after 4h at 17°C
. It is possible that the new protein released by freezing and thawing is not membrane-bound. Conversely, the residual new protein may be membrane-bound protein, some of which is rapidly transported into the motor axon during the period of protein labeling. The basis for this suggestion is our finding of a strong correlation between the residual new protein in cell bodies isolated from frozen, unprotected tissue and the new protein detected in their axons after 4h at 17°C ![]() .
.
11. How much acetylcholinesterase enters the motor axon each day?
This enzyme is found throughout the cell body, dendrites and axon of spinal motor neurons. Whether its main function in all of those areas is to hydrolyze the motor neuron’s neurotransmitter, acetylcholine, is not clear. We have used its hydrolytic activity as a marker for membrane-bound proteins to determine the amount of acetylcholinesterase (AChE) rapidly transported into the motor axon of the frog (Sinicropi et al., 1982). We found that 0.7 – 2.0 cell body equivalents of the enzyme entered the axon each day. The enzyme accumulates at the site of axonal injury, rising in activity within 3 days more than 3-fold relative to the non-injured ventral root. However, we found no evidence for its return to the cell body within the first few hours after injury, implying that this enzyme is an unlikely retrograde signal to the cell body that its axon has been injured ![]() .
.
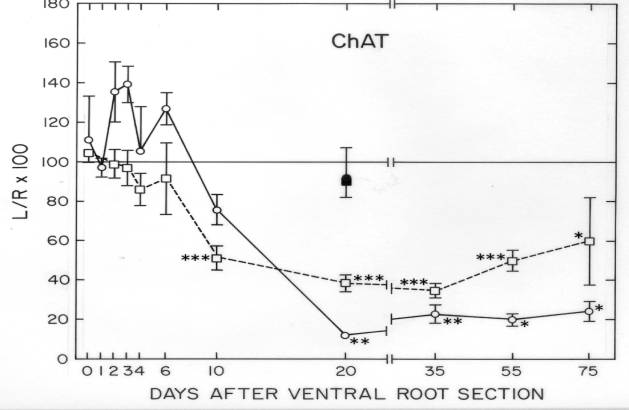
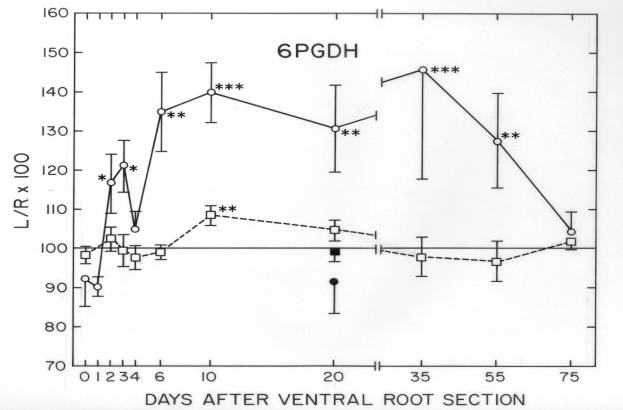
12. How much choline acetyltransferase and acetylcholinesterase do lumbar motor neuron cell bodies contain?
In our initial characterizations of isolated bovine motor neuron cell bodies (Weil et al., 1977), we evaluated their content of two cholinergic enzymes known to be present in motor neurons: choline acetyltransferase (ChAc), which synthesizes the cell’s neurotransmitter, acetylcholine, and AChE, which hydrolyses acetylcholine. The study served two main purposes: 1) identification of the isolated cell bodies as motor neurons – high activities of these two enzymes in the isolated cells would serve as one criterion that they were, in fact, motor neurons; and 2) determination of isolation conditions that maximize the retention of these two enzymes by the isolated cell bodies.
With respect to the first goal, we found relatively high activities of both enzymes in the cell bodies isolated from bovine lumbar and cervical spinal enlargements, consistent with their being spinal motor neurons. Not surprisingly, however, each enzyme represented very little (<0.01% by weight) of the total protein within the isolated cell bodies.
With respect to the second goal, we found that low pH, low ionic strength media, such as those described by Fonnum (1968), increased the recovery of the soluble enzyme, ChAc, 30-fold, but increased the recovery of the mostly membrane-bound AChE by only about 50%. Total cell body protein was also markedly increased during isolation by use of low pH, low ionic strength media (Weil et al., 1977).
13. What fraction of the acetyltransferase and acetylcholinesterase activity in the ventral gray matter is found in motor neuron cell bodies and their proximal dendrites?
In the same paper as in the preceding section (Weil et al., 1977), we estimated that as little as 10% of the ChAc and AChE activity in bovine lumbar and cervical ventral gray matter is confined to the motor neuron cell bodies and their proximal dendrites. This result was instructive, because histochemical and immunocytochemical analyses of spinal tissue sections show prominent localization of these two enzyme activities within motor neuron cell bodies. The explanation, we believe, is that the microscopic methods optimize the contrast in enzyme activity between the motor neurons and surrounding neuropil by stopping detection of activity while the weaker background activity of the neuropil is still low. When comprehensive measures of enzyme activity, such as ours, are performed, the background activity is accounted for and can be quite large in toto.
14. How early during development can choline acetyltransferase and acetylcholinesterase be detected in motor neurons?
Another difference between microscopic and in vitro analytical approaches to these two enzymes was encountered in a study done with my colleague, Dr. Paul Farel (Farel and McIlwain, 1983). We found that the sensitivity of our in vitro assay of AChE activity was higher than that of a standard histochemical assay for AChE. Specifically, we could detect the appearance of AChE activity in the ventral roots of developing frogs as early as stage V, while the histochemical procedure did not detect AChE activity until stage XII. Both AChE and ChAT (the abbreviation used for choline acetyltransferase in this paper) were present at the earliest larval stages analyzed, with ChAT increasing to much higher levels than AChE in adult frogs.
C. Normal Motor Neuron Size: Morphological and Biochemical Relationships
1. Why focus upon motor neuron size?
Three different issues drew our attention to motor neuron size. First, there is evidence for a particular vulnerability to disease in large neurons. “Large” neurons are, by our definition, those with a large total volume. Neurons with long, but thin axons (e.g., c fibers), or large cell bodies and short axons (Purkinje cells) are excluded from this definition. We and others before us have observed that in the early stages of ALS, large motor neurons appeared to be more vulnerable to the disease than small motor neurons (McIlwain, 1991). Later, middle-sized and small motor neurons become involved and die. ALS also appears to affect large, non-motor neurons, such as spinal border cells, neurons in Clarke’s column, and even large primary sensory neurons, as summarized in (Brock and McIlwain, 1984). A similar correlation has been found in inherited motor neuron diseases affecting dogs (Cork et al., 1989). Size-related vulnerability is less clear in transgenic SOD1 mice (Feeney et al., 2001; Morrison et al., 1998). Large motor neurons have also been reported to be the first to die in polio patients (Hodes et al., 1949). These data prompted us to study a number of structural and functional characteristics of motor neurons of different sizes as a means to understand the biological significance of those differences.
Secondly, the size of spinal motor neurons scales with whole body size (Hardesty, 1902). The larger the animal, the larger its average motor neuron size. This suggested to us that growth factors influencing whole body size, such as growth hormone and its chief mediator, insulin-like growth factor 1 (IGF-1), might also affect motor neuron size. We showed (Chen et al., 1997) that growth hormone does indeed increase motor neuron size, as well as the spinal cord and brain, during periods of early postnatal growth ![]() .
.
Thirdly, the size of plant and animal cells is often positively correlated with their DNA content (Cavalier-Smith, 1978). Large cells have several different possible strategies to increase their synthesis of proteins. They may increase the number of nuclei in each cell, as do skeletal muscle cells; increase the DNA content of their nuclei through polyploidy, as do some liver cells; through polyteny, as in insect larvae; or amplify some genes, such as those used to make ribosomal RNA. In addition, some cells, such as ova, can import RNA and thereby boost their protein synthetic capability. We wished to know which, if any, of these mechanisms is used by motor neurons. As described elsewhere ![]() , we found no evidence for polyploidy or polyteny, but could not rule out gene amplification in spinal motor neurons . We postulated that motor neurons may increase the transcribability of their DNA by unravelling the coiled DNA, thereby enlarging their nuclei and making them much less intensely stained by methylene blue and other basophilic dyes
, we found no evidence for polyploidy or polyteny, but could not rule out gene amplification in spinal motor neurons . We postulated that motor neurons may increase the transcribability of their DNA by unravelling the coiled DNA, thereby enlarging their nuclei and making them much less intensely stained by methylene blue and other basophilic dyes ![]() .
.
For these reasons, we embarked upon a series of investigations that examined structural characteristics of motor neurons in general, as well as structure-function relationships of large vs. small motor neurons.
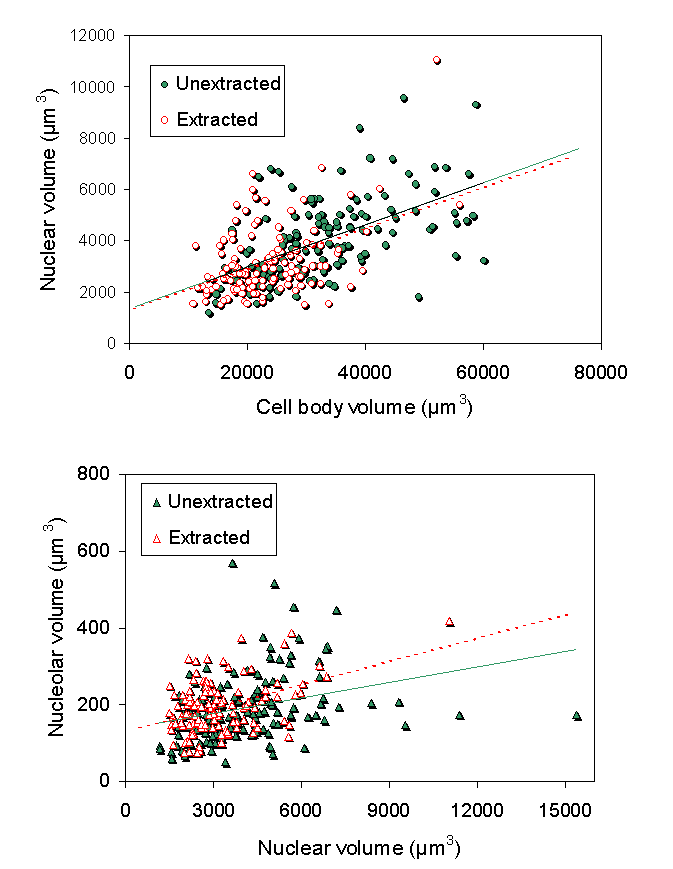
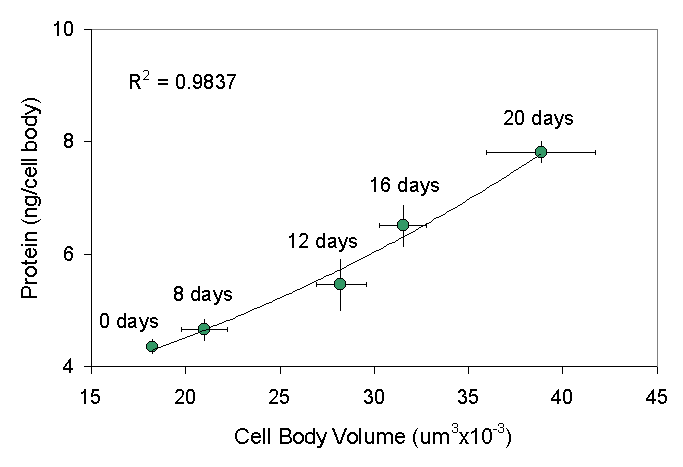
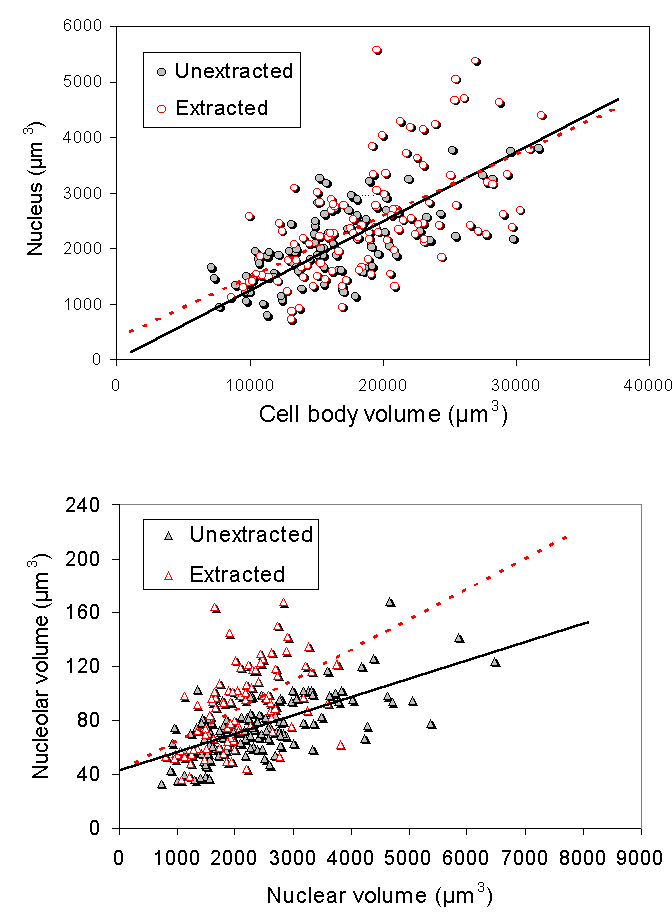
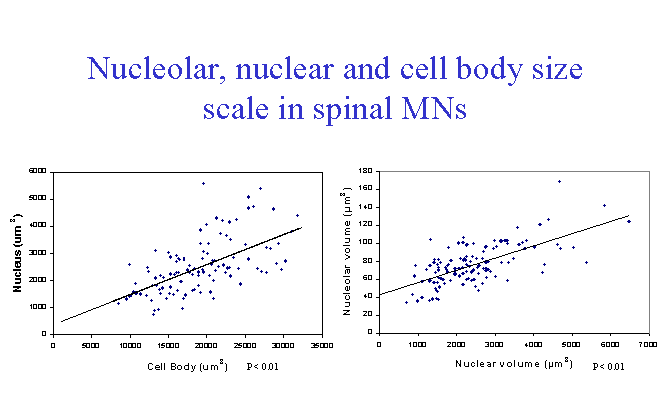
In our first study on nuclei isolated from bovine motor neuron cell bodies (McIlwain and Capps-Covey, 1976), we observed a wide range of nuclear sizes in motor neurons. When nuclear area was quantified in isolated motor neurons by morphometric methods, a strong positive correlation between nuclear and cell body area in motor neurons isolated from mice (Chen et al., 1997), frogs, rats and human beings (McIlwain, 1991). These size relationships are not isometric, but are allometric, with some degree of independence in nuclear and cell body size over the range of motor neuron sizes. For example, as one examines mature motor neurons of progressively larger size, cell body area increases faster than nuclear area in each of these animals. The range of normal cell body areas measured in isolated mouse motor neurons was close to the range of cell body areas reported for fixed tissue sections from the mouse lumbar spinal cord, although the average area of the unfixed cells was 10-15% larger (Chen et al., 1997). Nucleolar area is also positively correlated with nuclear and cell body area in isolated motor neurons. Volumetric measures of nucleolar, nuclear and cell body size were carried out in a later study on isolated frog motor neurons (McIlwain and Hoke, 2005), which again showed scaling among them over a five-fold range of cell body volumes ![]() .
.
3. Is the cytoskeleton responsible for maintaining these size relationships?
The persistent scaling of nucleolar and nuclear size with cell body size in spinal motor neurons stimulated our interest in the mechanism by which their sizes are linked. Studies by others on non-neurons (Fey et al., 1984) indicated that a nucleocytoplasmic lattice also might be responsible for maintaining nuclear and cell body size in motor neurons. This lattice or matrix can be isolated, using procedures devised for isolating the nuclear matrix. Since normal motor neurons vary in their size, we hypothesized that matrices isolated from motor neurons should exhibit the same size relationships between the nucleolus, nucleus and cell body as found in all our previous studies.
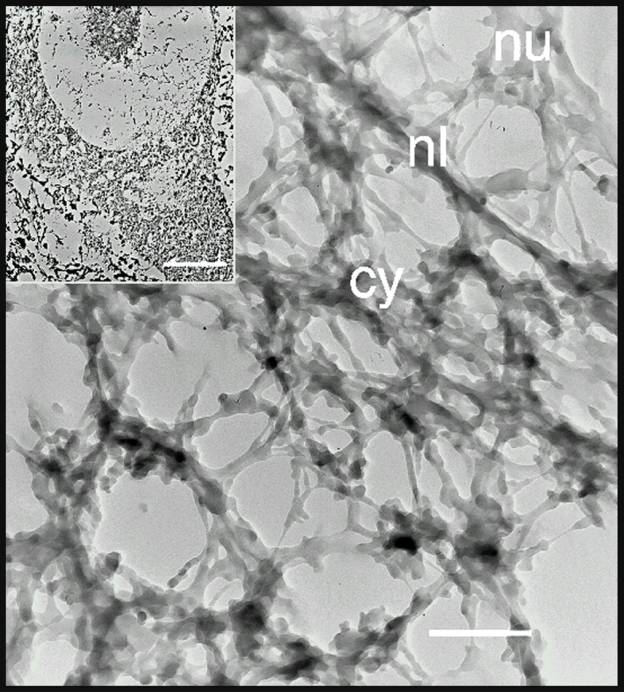
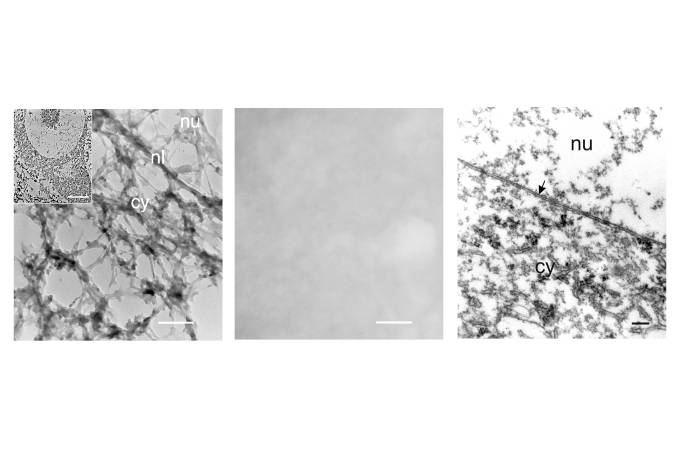
Both our electron microscopic and morphometric analyses by light microscopy supported this possibility. Using the matrix isolation procedure of Penman and his colleagues (He et al., 1990) on frog spinal cord, coupled with embedment-free electron microscopy, we found a lattice within spinal motor neurons that incorporated the nucleolus, nucleus, cell body and proximal dendrites into a single, integrated structure ![]() . The ultrastructure of the motor neuron cytoskeleton was comparable to that found in non-neuronal cells (He et al., 1990). It is a highly crosslinked network of filamentous proteins with electron dense particles – probably ribosomes – interspersed throughout the lattice. The filamentous struts of the lattice are tapered, suggesting the possibility of artifactual deposition of some protein onto the lattice. This matrix extends into the proximal dendrites of the motor neuron and we strongly suspect that it will be found throughout most of the extent of both the dendrites and axon. It may serve as a scaffold upon which other cytoskeletal proteins, such as neurofilaments, peripherin, tubulin and actin are organized in the cell.
. The ultrastructure of the motor neuron cytoskeleton was comparable to that found in non-neuronal cells (He et al., 1990). It is a highly crosslinked network of filamentous proteins with electron dense particles – probably ribosomes – interspersed throughout the lattice. The filamentous struts of the lattice are tapered, suggesting the possibility of artifactual deposition of some protein onto the lattice. This matrix extends into the proximal dendrites of the motor neuron and we strongly suspect that it will be found throughout most of the extent of both the dendrites and axon. It may serve as a scaffold upon which other cytoskeletal proteins, such as neurofilaments, peripherin, tubulin and actin are organized in the cell.
Embedment-free, unstained thin sections provide a very different view of the motor neuron cytoskeleton than conventional Epon-embedded, stained thin sections. The three-dimensional lattice seen when the embedding medium is removed is invisible when the embedding medium is present. Moreover, it differs greatly from the two-dimensional image of cells embedded in plastic ![]() , which masks much of the underlying filamentous structure. Penman (1995) emphasized the usefulness of embedment-free methods in understanding the architecture and function of the cytoskeleton.
, which masks much of the underlying filamentous structure. Penman (1995) emphasized the usefulness of embedment-free methods in understanding the architecture and function of the cytoskeleton.
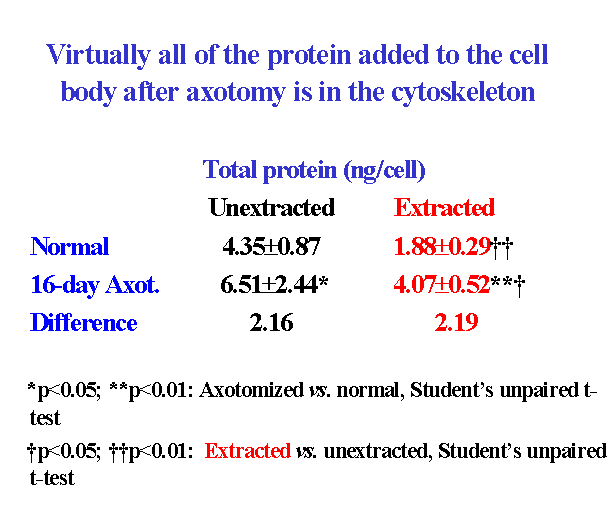
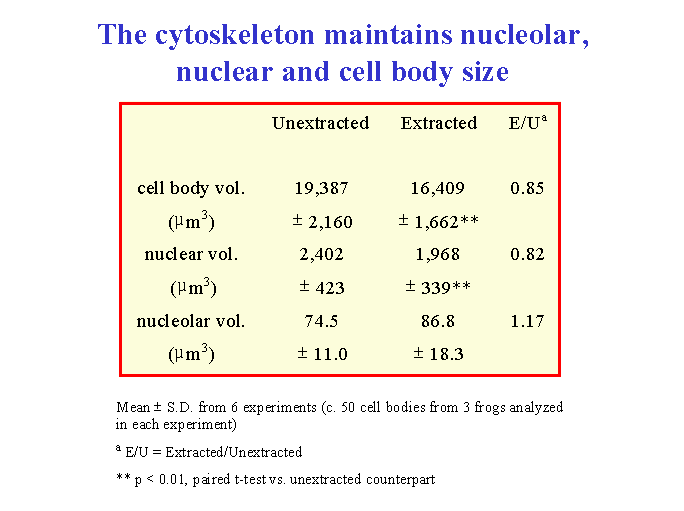
When isolated frog lumbar motor neurons were extracted and their cytoskeletons were analyzed morphometrically by interference contrast microscopy, the mean size of the nucleolus, nucleus and cell body in the cytoskeletons closely matched those of unextracted motor neurons ![]() . The nuclear position within the motor neuron cell body was also maintained in the isolated cytoskeleton. In addition, nucleolar, nuclear and cell body volume scaled with one another in the cytoskeleton in the same way as in unextracted motor neurons
. The nuclear position within the motor neuron cell body was also maintained in the isolated cytoskeleton. In addition, nucleolar, nuclear and cell body volume scaled with one another in the cytoskeleton in the same way as in unextracted motor neurons ![]() . From these data, we concluded (McIlwain and Hoke, 2005) that nucleolar and nuclear size and nuclear position are maintained in the motor neuron cell body by a filamentous lattice or matrix.
. From these data, we concluded (McIlwain and Hoke, 2005) that nucleolar and nuclear size and nuclear position are maintained in the motor neuron cell body by a filamentous lattice or matrix.
4. What are the mechanical properties of the cytoskeleton in motor neuron cell bodies?
The cell body cytoskeleton in spinal motor neurons contains less than one-half of the total cell body protein and is destroyed by trypsin. It has several unusual physical and chemical properties. Mechanically, it is very tough. Like the cells themselves, the matrix resists physical distortion of its form and size when being manipulated under the light microscope. A cell body attached to a glass slide by a single dendrite and tugged upon with a micropipette and mouth suction resists stretching and breaking. Occasionally, cell bodies are found that were severed by the nylon mesh ![]() during their isolation. The straight, cut edge of the cell body suggests the presence of a mechanically stiff cytoskeleton. These observations are consistent with those made in studies where the nucleus is removed from isolated frog motor neurons. In those studies (Sato et al., 1994), we were impressed with how difficult it was to disrupt isolated motor neurons and to liberate their nuclei by sonication. Moreover, after their nucleus had finally been removed, the cell bodies were often left with a hole in them where the nucleus had been. In general, we found that isolated motor neuron cell bodies are notably rigid structures.
during their isolation. The straight, cut edge of the cell body suggests the presence of a mechanically stiff cytoskeleton. These observations are consistent with those made in studies where the nucleus is removed from isolated frog motor neurons. In those studies (Sato et al., 1994), we were impressed with how difficult it was to disrupt isolated motor neurons and to liberate their nuclei by sonication. Moreover, after their nucleus had finally been removed, the cell bodies were often left with a hole in them where the nucleus had been. In general, we found that isolated motor neuron cell bodies are notably rigid structures.
5. What are the solubility properties of the cytoskeleton in motor neuron cell bodies?
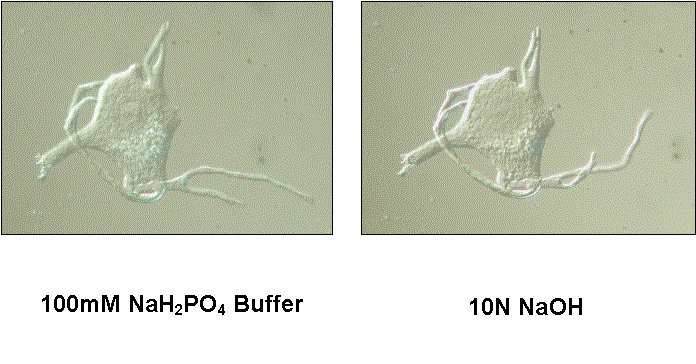
One of the unusual chemical properties of the cell body matrix is its ability to resist disassembly and solubilization by a variety of strong agents. This resistance to solubilization is not a consequence of the procedures used to minimize protein loss from motor neurons during their isolation, because motoneurons dissociated directly from spinal tissue and not subjected to the isolation procedures showed the same resistance. For example, directly dissociated human motor neuron cell bodies do not lose their form when exposed at room temperature to 10N NaOH, 8M urea, 10N HCl, 1% mercaptoethanol, 1% dithiothreitol, chloroform-methanol (2:1), 100% butanol or 100% ethanol. They did disappear when visualized with DIC optics and exposed to 1% SDS or 1% cetyltrimethylammonium bromide (CTAB), however. Whether the matrix was solubilized by these two ionic detergents was not clear, since we recovered 35% of the total cell body protein after exposure to 1% SDS in low-speed pellets, indicating a significant fraction of the total protein was not soluble in 1% SDS. We have not determined whether covalent crosslinks are present in this cytoskeleton. The striking mechanical and solubility properties of the cytoskeleton could conceivably play an important role in the inability of adult motor neurons to undergo mitosis.
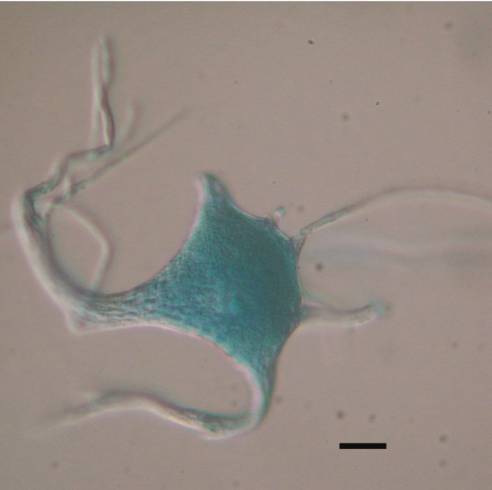
An intriguing and unexpected observation was made when motor neuron cell bodies isolated from frog, mouse or human spinal tissue were first stained with methylene blue and then exposed to 1% SDS or 1% CTAB. Those cell bodies did not disappear when observed by DIC, but maintained their form. Exposure to concentrations of SDS or CTAB as high as 10% at room temperature did not cause dissolution of the cell bodies. Human cell bodies exposed to methylene blue contained twice as much protein (9.2ng/cell) in the presence of 1% SDS than did cell bodies not exposed to methylene blue (4.5ng/cell). The mechanism by which methylene blue stabilizes the motor neuron cytoskeleton and prevents its dissolution by strong ionic detergents is unclear.
6. What are the major proteins in the cell body cytoskeleton?
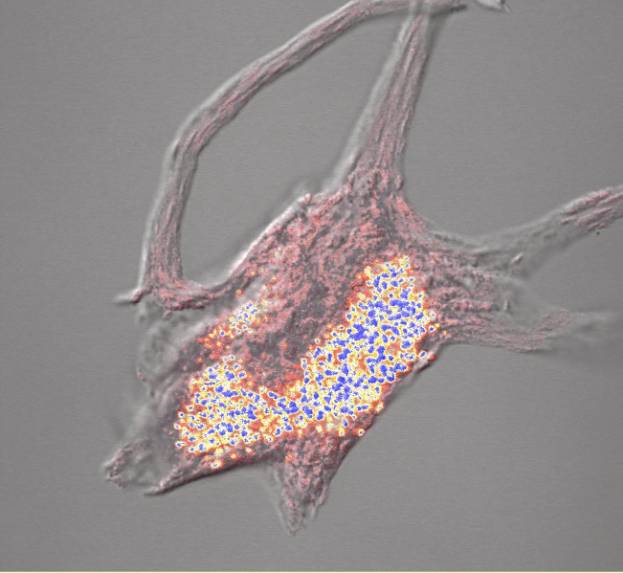
Studies on non-neuronal cells (Fulton et al., 1980) and on motor neurons (McIlwain and Hoke, 2005) suggest that intermediate filaments and ribosomal proteins may be abundant in the cell body cytoskeleton. To confirm the presence of ribosomes, we performed confocal imaging of cell body cytoskeletons isolated from human motor neurons stained with methylene blue, which binds to RNA. Human motor neurons contain well-formed Nissl bodies, which were easily visualized by light microscopy when stained with basophilic dyes. Confocal images of methylene blue-stained cytoskeletons isolated from normal human motor neuron cell bodies showed abundant Nissl bodies in the cell body and proximal dendrites ![]() . Although frog spinal motor neurons have less well organized Nissl bodies, cytoskeletons isolated from them also stain well with methylene blue. The RNA content of isolated cell body cytoskeletons has not been quantified by the methods used for unextracted motor neurons
. Although frog spinal motor neurons have less well organized Nissl bodies, cytoskeletons isolated from them also stain well with methylene blue. The RNA content of isolated cell body cytoskeletons has not been quantified by the methods used for unextracted motor neurons ![]() .
.
Further evidence that the RNA-rich cytoskeleton we isolate maintains motor neuronal size and shape is presented below ![]() . An interesting question arises: are the positions of ribosomes embedded within intersections of the cytoskeletal struts
. An interesting question arises: are the positions of ribosomes embedded within intersections of the cytoskeletal struts ![]() determined by genetic instructions that co-localize specific ribosomes and cytoskeletal junctions, thereby specifying size, shape and protein synthesis in each locale of the neuron?
determined by genetic instructions that co-localize specific ribosomes and cytoskeletal junctions, thereby specifying size, shape and protein synthesis in each locale of the neuron?
The two major types of intermediate filament in spinal motor neurons are the neurofilament triplet and peripherin. Jacomy et al. (1999) have reported that spinal motor neuron cell bodies in transgenic mice that are incapable of producing neurofilaments, because they lack the high and middle molecular weight neurofilament subunits, still retain their normal shape. Likewise, Larivière et al. (2002) found that transgenic mice lacking peripherin also have normal appearing motor neuron cell bodies. Although compensations for losses of the individual proteins could conceivably preserve cell body structure, these observations cast doubt on whether neurofilaments or peripherin are essential for the shape of normal motor neuron cell bodies.
Our efforts to determine by gel electrophoresis whether either of these intermediate filament proteins were present in the isolated cytoskeleton of motor neuron cell bodies were complicated by problems related to the solubility properties of the cytoskeleton alluded to above ![]() . Before we discovered that methylene blue rendered the cytoskeleton insoluble in strong ionic detergents, we performed 1-D and 2-D electrophoretic studies on extracted and unextracted cell bodies isolated from frog motor neurons. On 1-D polyacrylamide gels of unextracted cell bodies exposed to methylene blue, approximately 75% of the total silver stain was found in a background streak running the length of the sample lane. Only the streak appeared when extracted cell bodies were analyzed, consistent with our later discovery that methylene blue produced cell bodies that were not soluble in SDS. Two-dimensional polyacrylamide gels of unextracted frog cell bodies exposed to methylene blue had well-defined spot patterns (Sinicropi and McIlwain, 1983) with no observed correlate for the streaking seen on 1-D gels. In contrast, no silver-stained spots were found on 2-D gels of extracted cell bodies.
. Before we discovered that methylene blue rendered the cytoskeleton insoluble in strong ionic detergents, we performed 1-D and 2-D electrophoretic studies on extracted and unextracted cell bodies isolated from frog motor neurons. On 1-D polyacrylamide gels of unextracted cell bodies exposed to methylene blue, approximately 75% of the total silver stain was found in a background streak running the length of the sample lane. Only the streak appeared when extracted cell bodies were analyzed, consistent with our later discovery that methylene blue produced cell bodies that were not soluble in SDS. Two-dimensional polyacrylamide gels of unextracted frog cell bodies exposed to methylene blue had well-defined spot patterns (Sinicropi and McIlwain, 1983) with no observed correlate for the streaking seen on 1-D gels. In contrast, no silver-stained spots were found on 2-D gels of extracted cell bodies.
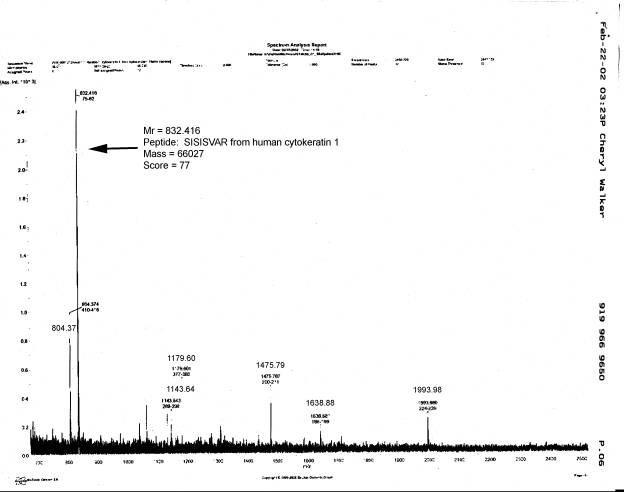
After the effects of methylene blue on cytoskeletal solubility were discovered, other experiments with a higher priority than these electrophoretic analyses kept us from returning to electrophoretic analyses of extracted cell bodies not exposed to the dye. Instead, we used mass spectrometry to analyze the proteins in isolated cell body cytoskeletons from human spinal motor neurons. The procedure used to prepare and analyze these samples involved the extraction of isolated cell bodies with 10N NaOH ![]() , rather than extraction by the procedure or He et al. (1990). Human motor neuron cell bodies extracted with 10N NaOH retain their normal size and shape
, rather than extraction by the procedure or He et al. (1990). Human motor neuron cell bodies extracted with 10N NaOH retain their normal size and shape ![]() . This procedural change was based upon our observation that human cell body cytoskeltons extracted with 10N NaOH contained an average of 6.1 ± 0.56 ng protein, while those extracted with Triton X-100 and high salt concentrations contained 9.5 ± 3.0 ng protein. Thus, NaOH removed about 53% of the total cell body protein, while the method of He et al. (1990) removed only 27.5% of the total protein. We do not know the reason for the lower extraction efficiency of the latter method for human vs. frog motor neurons.
. This procedural change was based upon our observation that human cell body cytoskeltons extracted with 10N NaOH contained an average of 6.1 ± 0.56 ng protein, while those extracted with Triton X-100 and high salt concentrations contained 9.5 ± 3.0 ng protein. Thus, NaOH removed about 53% of the total cell body protein, while the method of He et al. (1990) removed only 27.5% of the total protein. We do not know the reason for the lower extraction efficiency of the latter method for human vs. frog motor neurons.
Repeated MALDI-TOF analyses of the tryptic fragments from human motor neuron cell bodies were inconclusive. Only human cytokeratin 1 (K1) was detected by mass spectrometry in samples of cytoskeletons or unextracted cell bodies that contained 2-4 mg of protein ![]() . While it is possible that motor neurons, which are of ectodermal origin, contain cytokeratin 1, it is well known that this protein frequently contaminates mass spectrometric analyses of proteins. We found evidence that at least some of the cytokeratin we detected came from the sucrose solutions we used for isolating the cell bodies. Sucrose “blanks” that were processed through the same steps used to prepare the tryptic digests of the cell body cytoskeletons contained cytokeratin 1 by mass spectrometry. The contaminating protein could be detected on silver-stained 1-D gels and was present wherever sucrose was present, but was not seen in the other reagents we used to prepare motor neurons for mass spectrometry. Numerous attempts were made to circumvent this problem by testing different commercial sources of “ultrapure” sucrose and by using HPLC water and filtering the sucrose solutions twice with Millipore CX immersible filters to remove much of the contaminating protein from the sucrose. Nevertheless, all analyses of the cytoskeletal proteins yielded only cytokeratin 1.
. While it is possible that motor neurons, which are of ectodermal origin, contain cytokeratin 1, it is well known that this protein frequently contaminates mass spectrometric analyses of proteins. We found evidence that at least some of the cytokeratin we detected came from the sucrose solutions we used for isolating the cell bodies. Sucrose “blanks” that were processed through the same steps used to prepare the tryptic digests of the cell body cytoskeletons contained cytokeratin 1 by mass spectrometry. The contaminating protein could be detected on silver-stained 1-D gels and was present wherever sucrose was present, but was not seen in the other reagents we used to prepare motor neurons for mass spectrometry. Numerous attempts were made to circumvent this problem by testing different commercial sources of “ultrapure” sucrose and by using HPLC water and filtering the sucrose solutions twice with Millipore CX immersible filters to remove much of the contaminating protein from the sucrose. Nevertheless, all analyses of the cytoskeletal proteins yielded only cytokeratin 1.
It is unlikely that cytokeratin contaminating sucrose was abundant enough to prevent detection of other proteins in our samples. Almost all of the protein measured in micro Lowry assays of extracted cell bodies was cytoskeletal protein and was not cytokeratin from sucrose. Quantitative analyses of the sucrose solutions alone showed barely detectable amounts of protein, indicating that very little cytokeratin was present in them on a weight basis. Still, mass spectrometry detected only cytokeratin 1. Because the interconnected filaments of the cell body cytoskeleton may be covalently crosslinked, it is possible that trypsin cannot produce fragments of the filaments with masses less than 2500, thereby excluding them from analysis under our experimental conditions. We could demonstrate that, unlike intact cytoskeletal proteins, the peptide fragments released from the cytoskeletons by trypsin no longer pelleted in water at 13,000 x g for 10 min. However, 1-D PAGE of cell bodies extracted with 10N NaOH showed only cytokeratin 1 with silver staining, even when delivered to the gels in lysis buffer that was heated at 100 °C for 10 min and that contained as much a 100mM dithiothreitol. The fact that no other bands were detected anywhere on the gels suggested that the tryptic fragments were not soluble enough to enter the 1-D gels. It could not mean that only cytokeratin 1 is present in the cytoskeleton, because of the demonstrated presence of ribosomes in the isolated cytoskeleton ![]() .
.
Neither the molecular structure nor the composition of the filamentous matrix that determines the size and shape of motor neuron cell bodies is well understood. Whether the highly insoluble and cross-linked matrix we isolate contains cytoskeletal proteins unrecognized thus far in motor neurons and whether neurofilament protein or peripherin are components of the isolated cell body cytoskeleton is not yet known. Had the mass spectrometric methods we used been less expensive and available earlier in our research on motor neurons, we would have pursued these issues further.
7. Does the cytoskeleton cage lipofuscin granules?
An incidental observation made when monitoring cell bodies isolated from human spinal motor neurons and exposed to SDS by interference contrast indicated that lipofuscin granules are trapped within the interstices of the cytoskeleton. Lipofuscin granules are a prominent feature of adult human motor neuron cell bodies and are retained by the isolated cytoskeleton ![]() . We noticed that human cell bodies previously stained with methylene blue swell slightly after a few minutes when subsequently exposed to 10% SDS at room temperature. As the swelling appears, liposfuscin granules, which have average diameters of about 2.5 um (range 1.8-3.0 um, n = 16) and are insoluble in 10% SDS, begin to float away from the cell body. Even after depletion of most of the cell’s visible lipofuscin, the swollen cell body is still visible with interference contrast optics. This reproducible sequence of events led us to infer that lipofuscin granules are caged within the cytoskeletal lattice and can individually escape it as the lattice enlarges to pore sizes that exceed the diameter of the granule.
. We noticed that human cell bodies previously stained with methylene blue swell slightly after a few minutes when subsequently exposed to 10% SDS at room temperature. As the swelling appears, liposfuscin granules, which have average diameters of about 2.5 um (range 1.8-3.0 um, n = 16) and are insoluble in 10% SDS, begin to float away from the cell body. Even after depletion of most of the cell’s visible lipofuscin, the swollen cell body is still visible with interference contrast optics. This reproducible sequence of events led us to infer that lipofuscin granules are caged within the cytoskeletal lattice and can individually escape it as the lattice enlarges to pore sizes that exceed the diameter of the granule.
8. Do large motor neurons contain more nuclear DNA than small motor neurons?
Although we showed that the nuclei of normal spinal motor neurons contain diploid amounts of DNA on average ![]() , it remained possible that their DNA content might vary somewhat according to cell body size. Because nuclear and cell body size are positively correlated, the size of nuclei obtained from isolated motor neuron cell bodies can be used as an indication of the size of its parent motor neuron. Thus, simultaneous fluorescence activated flow cytometry and light scattering were used to determine the size and DNA content of individual, propidium iodide-stained nuclei obtained from isolated frog spinal motor neurons (Sato et al., 1994). While we found no evidence of polyploidy, there was a slight increase in the amount of propidium iodide-stained DNA in nuclei of increasing size. Whether this small increase represents an actual gain in DNA is not clear, since an equally plausible explanation is that as nuclear size increases, DNA unfolding (but not DNA content) increases, causing more of the propidium iodide to intercalate within the DNA. It is conceivable that ribosomal DNA, of which there are multiple copies in cells, could increase through gene amplification with increasing motor neuron size. Had an independent method for quantifying the DNA content of individual nuclei been widely available at the time of this study, we would have directly examined the question of whether there are slight increases in DNA content in larger nuclei from motor neurons.
, it remained possible that their DNA content might vary somewhat according to cell body size. Because nuclear and cell body size are positively correlated, the size of nuclei obtained from isolated motor neuron cell bodies can be used as an indication of the size of its parent motor neuron. Thus, simultaneous fluorescence activated flow cytometry and light scattering were used to determine the size and DNA content of individual, propidium iodide-stained nuclei obtained from isolated frog spinal motor neurons (Sato et al., 1994). While we found no evidence of polyploidy, there was a slight increase in the amount of propidium iodide-stained DNA in nuclei of increasing size. Whether this small increase represents an actual gain in DNA is not clear, since an equally plausible explanation is that as nuclear size increases, DNA unfolding (but not DNA content) increases, causing more of the propidium iodide to intercalate within the DNA. It is conceivable that ribosomal DNA, of which there are multiple copies in cells, could increase through gene amplification with increasing motor neuron size. Had an independent method for quantifying the DNA content of individual nuclei been widely available at the time of this study, we would have directly examined the question of whether there are slight increases in DNA content in larger nuclei from motor neurons.
9. Do large motor neurons synthesize more RNA than small motor neurons?
Conventional RNA radiolabeling methods do not distinguish among increased synthesis, decreased degradation and export of newly synthesized RNA from the nuclei. In order to obtain a more specific measure of RNA synthesis, we utilized a “nuclear run-on” procedure in which newly synthesized RNA was unable to leave the DNA on which it was being made. A strong positive correlation was found between nuclear volume and RNA labeling was observed in nuclei isolated from frog motor neuron cell bodies labeled under run-on conditions (Sato et al., 1994). While we favor the view that this size-dependent increase in RNA synthesis is a manifestation of the highly uncoiled and dispersed nature of the euchromatin in large nuclei, it is still possible that slight increases in nuclear DNA could be a contributory factor. Whatever its mechanism, the size-dependent increase in transcription is consistent with the long-held view that large cells require higher rates of RNA and protein synthesis to sustain their large size.
10. Do large motor neurons synthesize more protein than small motor neurons?
Unlike the nuclear run on assay for RNA synthesis, there is no assay that strictly measures the rate of protein synthesis, while controlling for changes in protein degradation or export from the motor neuron cell body. This means that the plethora of studies using labeling of proteins by radioactive amino acid precursors can tell one only whether a change has taken place in the rate of synthesis, degradation or export of a newly made protein. With this significant limitation, we measured the in situ incorporation of 3H-L-leucine into the proteins of motor neuron cell bodies, prior to their isolation from the lumbar spinal cord of frogs (McIlwain and Hoke, 1994). We found a clear increase in protein labeling with increasing cell body size of about the same magnitude (2-3 fold) as the size-dependent increase in RNA synthesis observed in frog motor neuronal nuclei. These data, along with other studies on injury-induced increases in transcription, protein labeling, and cell body size in frog motor neurons, move us closer to the unsurprising, but still not rigorously confirmed conclusion that large motor neurons synthesize more protein than small ones.
11. Does growth hormone play a role in motor neuron size?
Since growth hormone is known to enhance body size during postnatal growth of many animals, we suspected that it may enhance motor neuron size, which scales with body size. To test this possibility, we (Chen et al., 1997) analyzed the size of lumbar motor neurons in transgenic mice that overexpress human growth hormone (hGH) and also in transgenic mice that overexpress its chief mediator, insulin-like growth factor (IGF-1). We found that motor neurons in 58 -118 day-old mice overexpressing hGH had 30 % larger cell body areas than did their littermate, gender-matched controls, and larger nucleolar (33%) and nuclear areas (28%) as well. Body size in young adult transgenic mice expressing hGH was increased 80% and brain and spinal cord weight was elevated by 12% and 35%, respectively. In contrast, transgenic mice overexpressing human IGF-1 showed no increase in motor neuron size, although their body, brain and spinal cord size was enlarged by 37%, 54% and 71%, respectively. These experiments did not rule out a growth effect of IGF-1 on motor neurons, since elevation of circulating IGF-1 depresses GH production by the pituitary through a negative feedback mechanism, leaving open the possibility that GH and IGF-1 act synergistically to enlarge motor neurons in the hGH mice. We did not determine the extent to which motor neuron size depends upon GH. However, we estimated that endogenous GH may be responsible for about 10% of mature brain weight and about 24% of mature spinal cord weight in one line of mice (McIlwain et al., 2004).
12. Does growth hormone help to maintain motor neuron size in adult animals?
There is evidence for age-dependent atrophy of the human spinal cord (Kameyama et al., 1994). There is also evidence for a sharp decline in circulating GH levels in human beings after the end of postnatal growth and into adulthood (Hammerman, 1987). Given the data above for a trophic effect of GH on the size of the mouse spinal cord and motor neuron, it is possible that the decline in GH with age in human begins could cause a decline in motor neuron size with age. If it does, then the decline in circulating GH during adulthood might play a role in age-dependent neuromuscular dysfunction and perhaps even in the late onset of some motor neuron diseases. Thus, we sought a way to interfere experimentally with circulating GH after the period of postnatal CNS growth ends in mice as a means to determine whether motor neuron size depends upon GH during adult aging.
As a first step toward this goal, we focused upon a mouse line that expresses a growth hormone antagonist (GHA) transgene (Chen et al., 1994). The protein product of this transgene, a mutant hGH, had been shown to competitively inhibit the action of endogenous mouse GH at the level of the GH receptor, inhibiting body growth postnatally. If this mouse line were first shown to have smaller than normal spinal motor neurons, then we were interested in making an inducible form of the GHA transgene that could be turned on and off during adulthood, after the CNS has reached its normal size.
We were able to demonstrate that the GHA transgenic mouse has a smaller brain and spinal cord than its littermate, gender-matched controls (McIlwain et al., 2004). However, our initial morphometric studies on the areas of lumbar motor neuron cell bodies showed no significant decrease in size. It is possible that further analyses may detect a decrease in the size of lumbar motor neurons in the GHA mouse. Instead of pursuing this possibility, we opted to compare the growth rates of the brain, spinal cord and body during the postnatal growth in control and GHA mice. We found that GHA strongly inhibits body weight up until about postnatal day 50, after which its inhibitory effects are lost, and body weight in GHA mice “catches up” with that of littermate, gender-matched controls. We also found that GHA inhibits the magnitude and velocity of postnatal brain and spinal cord growth, the latter much more and for a longer duration into adulthood than the former. No “catch up” growth was observed for the brain and spinal cord during adulthood. Since GHA inhibits GH action, one can infer from these data that GHdifferentially affects the magnitude, velocity and duration of postnatal growth of the brain, spinal cord and body.