Mission and Accomplishments
The University of North Carolina School of Medicine created the Gene Therapy Center in 1993 with the goal of providing an intellectual environment for a diverse community of research scientists dedicated to basic and translational research. The primary mission is to focus on the development of novel gene delivery systems and execute translational research for a patient population affected by genetic disorders. Research in the Center has focus on the molecular biology of viral (adeno- associated virus (AAV), Lenti, and Adenovirus (Ad)) and non-viral vectors in order to exploit the unique features of these delivery systems for use in basic research with eventual goal of human gene therapy. Continued efforts in understanding the mechanism of cell entry, persistence, and immune response are being pursued at basic science level in order to create more efficient gene transfer vectors.
As a result of state-of-the-art capabilities of Center scientists, the Gene Therapy Center provides important resources to academic investigators through two specialized core facilities created to support preclinical and clinical gene therapy studies. These facilities, the Vector Core and the Human Applications Laboratories (HAL), were created to ensure that investigators would have promising gene vectors available in the quality and quantities needed for preclinical or clinical studies.
The ultimate goal of the Gene Therapy Center is to facilitate the progression and translation of gene therapy research from the laboratory bench into Phase I clinical trials for the treatment of human disease. As seen on the cover of Science Dec 2013 “The breakthrough of the year” is gene therapy for T-cell that signals again that this area of research is coming of age.
In light of this point and the UNC mission objective developing “bench to bedside” therapeutics, the UNC GTC has initiated and completed a number of seminal Phase I gene transfer studies including:
 |
 |
|---|---|
| 1) Completion of first AAV gene delivery study for neurological disorder (Canavan Sic Trans-2012). | 2) Completion of the first AAV gene delivery study for Duchenne Muscular Dystrophy (NEJM 2010) |
 |
 |
|---|---|
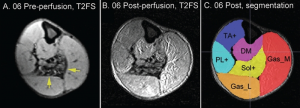
| 3) Completion of first Limb Infusion Safety Study for DMD (Mol Ther 2012) | 4) Initiation of AAV gene transfer Phase I study for Hemophilia B patients using UNC modified FIX transgene |