Examining the role of dietary fatty acids – Eric Klett, MD
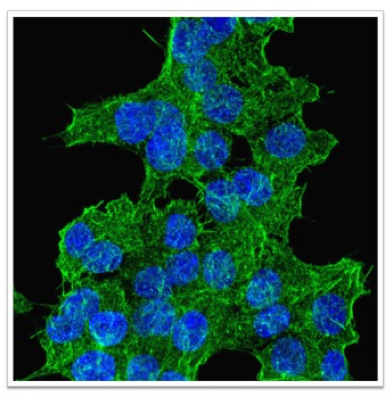
Dr. Eric Klett is a physician-scientist whose research interest is in the role of dietary fatty acids, specifically polyunsaturated fatty acids (PUFAs), and their effects on the development of diabetes mellitus.
The Klett lab, using pancreatic beta-cell and animal models, examines the role of one of the rate-limiting enzymes in lipid metabolism on pancreatic beta-cell function.
Exposing beta-cells to ω-6 PUFAs (arachidonate or linoleate) impairs glucose-stimulated insulin secretion. Further, feeding mice diets enriched in ω-6 PUFAs impairs insulin secretion in respond to a glucose load leading to hyperglycemia.
In collaboration with the UNC Microscopy core, Dr. Klett’s lab is using real-time calcium flux and actin polymerization to determine the effects of dietary PUFAs on insulin secretion. Dr. Klett’s focus is to determine the molecular mechanisms underlying these findings in hopes of developing dietary or pharmacologic interventions to improve the treatment of patients with diabetes mellitus.
Bone remodeling and the effects of exercise and mechanical force – Janet Rubin, MD
Dr. Janet Rubin investigates the controls over bone remodeling, in particular exercise and mechanical force effects on the cell cytoskeleton.
In collaboration with scientists at the Mayo Clinic we have been studied how mechanical force changes the internal structure of the nucleus. Several publications exemplify this work:
- Sen B, Uzer G, Samsonraj RM, Xie Z, McGrath C, Styner M, Dudakovic D, van Wijnen AJ, Rubin J 2017 Intranuclear actin structure modulates mesenchymal stem cell differentiation, Stem Cells, 35(6):1624-1635
- Samsonraj RK, … Rubin J, van Wijnen AJ 2018 Osteogenic programming of adipose-derived mesenchymal stem cells using a fungal metabolite that suppresses the Polycomb protein EZH2, Stem Cell Translational Medicine 7(2):197-209
Our recent post-doctoral fellows, now assistant professors at Boise State (Uzer) and University of Indiana (Thompson) have investigated the role of mechanical force in regulating b-catenin and RhoA action:
- Uzer G, Uzer B, Sen B, Xie Z, Birks S, Olcum M, McGrath C, Styner M, Rubin J 2018 SUN-mediated mechanical LINC between nucleus and cytoskeleton regulates βcatenin nuclear access, Journal Biomechanics 74:32-40.
- Thompson WR, Yen S, Uzer G, Styner M, Sen B, Xie Z, Burridge, Rubin J 2018 Larg/ARHGAP18 cycling controls RhoA activity and MSC response to environmental factors, Bone. 107:172-180
Dr. Rubin’s lab recently published a case detailing an important side effect of iron administration, FGF23 based rickets, which was written with a medical intern at UNC (Klein):
- Klein KR, Asaad S, Econs M, Rubin J 2018 Severe FGF23 based hypophosphatemic osteomalacia due to ferric carboxymaltose administration, BMJ Case Rep Jan3 doi: 10.1136/bcr-2017-222851
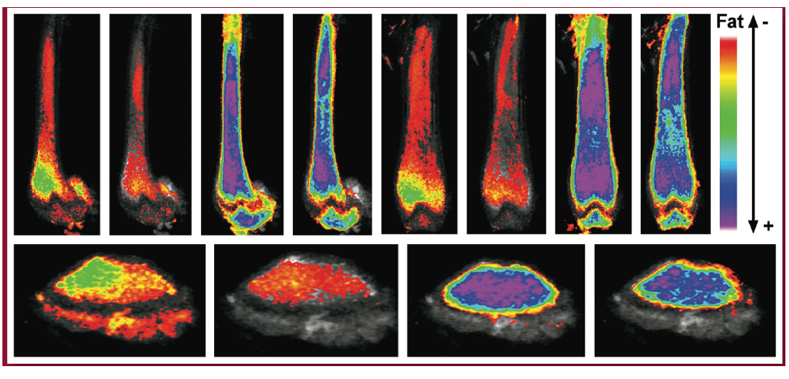
Quantification of marrow fat using image analysis – Maya Styner, MD
Dr. Maya Styner is a physician-scientist with an interest in improving our mechanistic understanding of how exercise expands bone quality and quantity, with an emphasis on the exercise effect on bone-fat (also known as marrow adipose tissue, MAT). Dr. Styner’s work is pertinent to her clinic population, who suffer from osteoporosis and related fractures. The lab’s work on exercise, dietary and pharmacologic regulation of marrow fat was the first in the field to shed light on a potential function of this fat depot as an energy store, deepening an understanding of marrow fat and its relationship to bone health.
Her laboratory has additionally validated two novel methods (applying small animal μCT and MRI) to quantify marrow fat volumetrically via advanced image analysis methodology, in collaboration with UNC Biomedical Research Imaging Center and image analysis experts. The improved quantification of marrow fat increases the rigor of future studies that aim to elucidate the purpose of this fat depot. Dr. Styner currently collaborates with UNC metabolism experts to study marrow fat in a rare fat-less disorder (congenital generalized lipodystrophy) as well as to improve our understanding of the metabolic function of this fat depot.
UNC Diabetes Care Center Clinical Trials Unit

M. Sue Kirkman, MD
 Dr. Sue Kirkman is the medical director of the UNC Diabetes Care Center’s Clinical Trials Unit, which consists of five investigators, five study coordinators, and three research assistants conducting 15-20 trials in type 1 and type 2 diabetes and in obesity. Unit investigators have published over 80 papers since 2017.
Dr. Sue Kirkman is the medical director of the UNC Diabetes Care Center’s Clinical Trials Unit, which consists of five investigators, five study coordinators, and three research assistants conducting 15-20 trials in type 1 and type 2 diabetes and in obesity. Unit investigators have published over 80 papers since 2017.
In the fall of 2017, Dr. Kirkman became co-PI of a CDC-funded grant to better distinguish between type 1 and type 2 diabetes in national surveillance systems. Currently, the data sources that CDC relies upon to monitor trends in diabetes prevalence and incidence do not reliably distinguish between types of diabetes, and it is unclear whether all people with diabetes know what type they have. Under current surveillance, important trends in incidence, severity, and outcomes of people with type 1 diabetes are being lost in the epidemic of the much more prevalent type 2 diabetes. Dr. Kirkman’s work has involved extensive phenotyping of 2500 adults with diabetes in the UNC Healthcare system, using data fields from the Carolina Data Warehouse for Health and additional information abstracted from chart notes. In the upcoming year of the project, UNC researchers and partners at Westat, will develop a phone survey and field it to the same cohort of patients. Survey responses will be compared against each patient’s “gold standard” type, allowing survey question validation. Dr. Kirkman’s study will contribute important information to the CDC that may eventually allow better surveillance of both types of diabetes. This is important to support type-specific analyses of morbidity, mortality, medical care costs, and health-related quality of life.
Beginning in January 2018, Dr. Kirkman became chair of the National Diabetes Education Program (NDEP), a joint initiative of NIDDK and the CDC. NDEP works collaboratively with its partners at the federal, state, and local levels to improve the treatment and outcomes for people with diabetes, promote early diagnosis, and prevent or delay the onset of type 2 diabetes. Dr. Kirkman is site principal investigator of several clinical trials including the NIDDK-funded GRADE study, for which she also serves as co-chair of the Outcomes Committee.
John Buse, MD, PhD
 Dr. John Buse participates in the UNC Diabetes Care Center’s Clinical Trial Unit and collaborates with investigators in the Schools of Medicine, Public Health, Pharmacy, and the Joint Department of Biomedical Engineering as well as multiple multicenter trials. Highlights in 2018 include receiving a notice of award in April refunding the NC Translational and Clinical Sciences Institute (NC TraCS) for 5 years with a Clinical and Translational Sciences Award of $58.1 million from the National Institutes of Health. In October 2018, a one year effort that Dr. Buse co-chaired to revise the American Diabetes Association and European Association for the Study of Diabetes guidance on the management of type 2 diabetes was published. A systematic evaluation of the literature since 2014 informed new recommendations including additional focus on lifestyle management, weight reduction and diabetes self-management education and support. With regards to medication management, for patients with clinical cardiovascular disease, treatment with an SGLT2 inhibitor (e.g., liraglutide) or a GLP-1 receptor agonist (e.g., empagliflozin) with proven cardiovascular benefit is recommended. For patients with clinical heart failure or chronic kidney disease, treatment with an SGLT2i with proven benefit is recommended. GLP-1 receptor agonists are generally recommended as the first injectable medication, before insulin.
Dr. John Buse participates in the UNC Diabetes Care Center’s Clinical Trial Unit and collaborates with investigators in the Schools of Medicine, Public Health, Pharmacy, and the Joint Department of Biomedical Engineering as well as multiple multicenter trials. Highlights in 2018 include receiving a notice of award in April refunding the NC Translational and Clinical Sciences Institute (NC TraCS) for 5 years with a Clinical and Translational Sciences Award of $58.1 million from the National Institutes of Health. In October 2018, a one year effort that Dr. Buse co-chaired to revise the American Diabetes Association and European Association for the Study of Diabetes guidance on the management of type 2 diabetes was published. A systematic evaluation of the literature since 2014 informed new recommendations including additional focus on lifestyle management, weight reduction and diabetes self-management education and support. With regards to medication management, for patients with clinical cardiovascular disease, treatment with an SGLT2 inhibitor (e.g., liraglutide) or a GLP-1 receptor agonist (e.g., empagliflozin) with proven cardiovascular benefit is recommended. For patients with clinical heart failure or chronic kidney disease, treatment with an SGLT2i with proven benefit is recommended. GLP-1 receptor agonists are generally recommended as the first injectable medication, before insulin.
With regards to new studies, Dr. Buse leads the SOUL trial, a cardiovascular outcome trial of oral semaglutide as well as other trials in type 2 diabetes of SGLT2 inhibitors and GLP-1 receptor agonists. In type 1 diabetes, he is leading trials of a new class of oral antihyperglycemic medication (glucokinase activator). In collaboration with Duke, Wake Forest University and NC A&T, Dr. Buse is working to establish a NC Diabetes Research Center encouraging collaborative work between the four institutions with UNC taking the lead in advanced clinical study methods.
Laura A. Young, MD, PhD
 Dr. Laura Young’s career as a clinical investigator focused on behavioral and complimentary approaches to diabetes management reached new heights. Along with co-PI Katrina Donahue from Family Medicine, she led the MONITOR trial, one of the first PCORI funded trials to be completed. Published in JAMA Internal Medicine, the trial has received wide recognition and acclaim for demonstrating that in patients with type 2 diabetes not treated with insulin, routine glucose monitoring or advanced monitoring linked to tailored messaging did not improve glycemic control versus no monitoring. This study was also one of the first at UNC to demonstrate our health care systems capabilities to conduct large scale pragmatic trials on time and within budget with high levels of patient retention and little missing data across the region. She provided leadership in the design of a suite of national studies of continuous glucose monitoring (CGM) and now serves as site PI for the WISDM (Wireless Innovation for Seniors With Diabetes Mellitus) study aimed to evaluate the efficacy of CGM particularly to reduce hypoglycemia (time spent with glucose <70 mg/dl) in elderly patients with type 1 diabetes.
Dr. Laura Young’s career as a clinical investigator focused on behavioral and complimentary approaches to diabetes management reached new heights. Along with co-PI Katrina Donahue from Family Medicine, she led the MONITOR trial, one of the first PCORI funded trials to be completed. Published in JAMA Internal Medicine, the trial has received wide recognition and acclaim for demonstrating that in patients with type 2 diabetes not treated with insulin, routine glucose monitoring or advanced monitoring linked to tailored messaging did not improve glycemic control versus no monitoring. This study was also one of the first at UNC to demonstrate our health care systems capabilities to conduct large scale pragmatic trials on time and within budget with high levels of patient retention and little missing data across the region. She provided leadership in the design of a suite of national studies of continuous glucose monitoring (CGM) and now serves as site PI for the WISDM (Wireless Innovation for Seniors With Diabetes Mellitus) study aimed to evaluate the efficacy of CGM particularly to reduce hypoglycemia (time spent with glucose <70 mg/dl) in elderly patients with type 1 diabetes.
Katherine Bergamo, RN, MSN, FNP-C
 In addition to her clinical care of outpatients with diabetes, Research Instructor Kate Bergamo is an investigator in the clinical trials unit and serves as UNC PI of the CGM Intervention in Teens and Young Adults with T1D (CITY) trial, funded by the Jaeb Center for Health Research.
In addition to her clinical care of outpatients with diabetes, Research Instructor Kate Bergamo is an investigator in the clinical trials unit and serves as UNC PI of the CGM Intervention in Teens and Young Adults with T1D (CITY) trial, funded by the Jaeb Center for Health Research.
People with type 1 diabetes in the age group of “transition” between pediatric and adult care often have lapses in care, poor glycemic control, and high risk of severe hypoglycemia. The randomized CITY trial seeks to assess the impact of continuous glucose monitoring on hypoglycemia rates, HbA1c, and quality of life in young adults who have not previously used this technology.
Sriram Machineni, MD
 The clinical trial group in the division of endocrinology diversified its efforts this year with the addition of Dr. Sriram Machineni, an obesity medicine specialist recruited from the Massachusetts General Hospital, whose practice and research leverages the spectrum of responses that essentially all clinical interventions for obesity are associated with. He has initiated clinical trials of weight loss medications to examine efficacy, safety and cardiovascular outcomes. He is a part of the transdisciplinary team which forms the Heterogeneity in Obesity Creativity Hub that is working on precision research and treatment approaches for obesity. The hub is funded by the UNC Creativity Hub initiative from the Office of the Vice Chancellor for Research. He is also participating in another large collaboration led from the School of Pharmacy to better characterize clinical responses to bariatric surgery and exploring potential microbiome based therapies to manage type 2 diabetes, funded by the Eshelman Institute for Innovation.
The clinical trial group in the division of endocrinology diversified its efforts this year with the addition of Dr. Sriram Machineni, an obesity medicine specialist recruited from the Massachusetts General Hospital, whose practice and research leverages the spectrum of responses that essentially all clinical interventions for obesity are associated with. He has initiated clinical trials of weight loss medications to examine efficacy, safety and cardiovascular outcomes. He is a part of the transdisciplinary team which forms the Heterogeneity in Obesity Creativity Hub that is working on precision research and treatment approaches for obesity. The hub is funded by the UNC Creativity Hub initiative from the Office of the Vice Chancellor for Research. He is also participating in another large collaboration led from the School of Pharmacy to better characterize clinical responses to bariatric surgery and exploring potential microbiome based therapies to manage type 2 diabetes, funded by the Eshelman Institute for Innovation.