The Top 10 HIV Clinical Developments of 2018
by David Alain Wohl, MD Professor, Division of Infectious Diseases, The University of North Carolina at Chapel Hill Director, North Carolina AIDS Training and Education Center Site Leader, The University of North Carolina Chapel Hill AIDS Clinical Research Site
An edited version of this report can be found at The Bodypro.com: http://www.thebodypro.com/content/81540/top-10-hiv-clinical-developments-of-2018.html?ic=tbphnews
It’s the beginning of the end. Not in some apocalyptic way but, rather in how we think about the prevention and management of HIV infection. A tea-leaf reader of the stories that made heads turn this past year could reasonably predict that the antiretroviral regimens of tomorrow will come in pairs, be delivered via a route other than the gastrointestinal tract, or both. The next great leap in HIV prevention will also look to novel ways of getting drugs where they need to be when they need to be there. Until then, we are still stuck trying to get providers to prescribe PrEP and those who can benefit from it to take it – all we are saying is give PrEP a chance.
Less mutable are the racial and socioeconomic influences in HIV outcomes. Inequities in HIV care are intractable and will be as long as society is stacked to shower preference and privileges on some, while thwarting and threatening others. The advocacy that is woven into the fabric of HIV care has supported great progress –the Ryan White Care Act, and in some places, the Affordable Care Act, have ensure access to health care for many living with or at risk of HIV infection. But, these efforts mitigate and do not obviate the inherent injustices that make the difference between staying uninfected or undetectable.
Any recap of where we are really is a preamble to speculation about where we are going. Beyond the innovations in medications, the year closed with a sour taste of things to come from a physicist in China who appears to believe he is servicing humanity by messing with it at its most basic level to fix a problem that can be solved more compassionately and ethically in other ways. That this scientist chose, among the options, to re-engineer the genes of babies to make them resistant to the HIV their father carries, speaks to an ignorance and fear that no tweaking of a molecule can cure.
- GEMINI – A Sign of the Rise of Two -Drug Therapy
The concept of a three-drug minimum for HIV therapy is rooted in a sequentialism that emerged with the dawning of antiretroviral treatment: one drug – nothing, two drugs – meh, three drugs – bingo! Over the years, in an effort to avoid peskier antiretrovirals or to reduce cost, some researchers have tinkered with alternatives but, these generally went nowhere (at least in the US). Since the days of the first protease inhibitor trial, triple-drug therapy has dominated.
But apostasy is at hand, and like all movements, antiretroviral minimalism started small and then grew. Pilots involving a handful pf participants fed bigger studies and then these led to randomized trials. Demonstrating that two-drugs could maintain HIV suppression planted the dual-therapy flag, and in late 2017 a fixed dose combination of rilpivirine (RPV) and dolutegravir (DTG) was approved by the US FDA as a two-drug switch option. However, maintaining viral suppression is one thing, achieving it another. Incrementally, a series of investigations that started with the toe-in-the-water PADDLE study, introduced us to the unlikely couple of the DTG and the cherry-on-top antiretroviral, 3TC. That this combination could succeed where super-power duos, such as an integrase inhibitor plus a boosted protease inhibitor, did not shows how match-making antiretrovirals, can be as complicated and unpredictable as match-making people.
This leads us to the results of the GEMINI-1 and -2 trials and unassailably the biggest HIV story of the year. These identical twin, phase III trials compared an initial two-drug regimen of DTG and 3TC against a three-drug combination of tenofovir disoproxil fumarate (TDF) and FTC plus DTG.1Large and double-blind trial, the results of these trials were heavily anticipated. In fact, before the results were first presented in a sweltering Amsterdam at the International AIDS Society Meeting, betting types were already handicapping the two-drug regimen, weighing how many virologic failures with drug resistance would the treatment community tolerate in this arm before declaring it dead-on-arrival.
As it turns out, such risk-benefit calculating was unnecessarily pessimistic. In the DTG+3TC arm a more than reassuring 91% had no HIV RNA detected in their blood at week 48, statistically no different than the 93% of those randomized to TDF/FTC + DTG (difference: -1.7%; 95% CI: -4.4% to 1.1%).And, for all that hand-wringing about barrier to resistance, the emergence of viral resistance to any of the study drugs was not observed in any participants. Drop the mic.
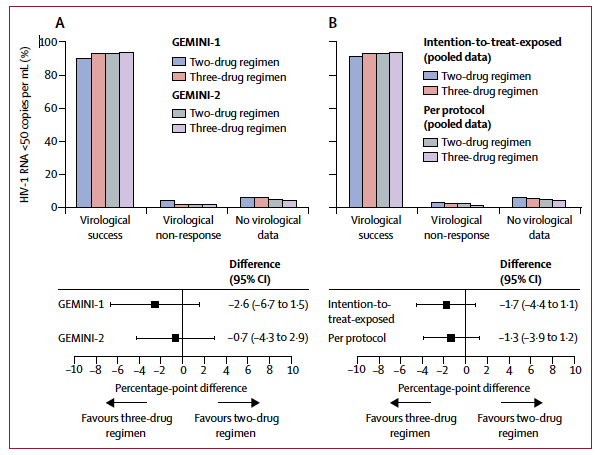
Subgroup analyses of minimal antiretroviral regimens have revealed unpleasantness in the past, and are important to this tale. In the GEMINI trials, there were no differences between the study arms among those entering with plasma HIV RNA levels above and below 100,000 copies/mL. However, 79% (50 of 63 participants) of those in the two-drug arm who had a baseline CD4+ cell count below 200 cells/mm3had a week 48 viral load that was undetectable compared to 93% (51 of 55 participants) of those entering with counts below this level assigned the three-drug regimen. The study team explained this away by noting how most, but not all, of those with CD4+ cell counts below 200 cells/mm3counted as failures in the DTG+3TC arm discontinued treatment for reasons unrelated to drug efficacy, such as adverse events not considered by the investigator to be treatment related, protocol violations, loss to follow-up, consent withdrawal, and change in ART. Most of these seem to be unrelated to efficacy but, the FDA snapshot algorithm cares not for excuses and while one could fully understand the motivation to explain the lower rate of success for DTG+3TC in the less than 200 cells/mm3stratum, traditionally in clinical trials there has been little attempt to do so.
The Bottom Line:Living as we are in an all-bets-are-off world where the previously unimaginable (not to say, the unacceptable) are commonplace, it has become difficult to be genuinely surprised. Yet, in the small corner of the universe that we call the field of HIV therapeutics only the arrogant or the visionary could believably claim to not be blown away by the success of DTG+3TC.
We have been flirting with pared down HIV treatment, currently life-long in duration, for years. But, what started with a bit of starry-eyed chit-chat over drinks has now bloomed into a full-on affair. While some caution against complete commitment to DTG+3TC as a first-line therapy option until longer-term data are gathered and this strategy is stress-tested in more diverse populations of people living with HIV infection, we have to accept that we do clinical trials for a reason and the GEMINI results are striking.
Understandably, it is hard to part from the tried and true, and absent a clear signal of longer-term toxicity of current triple therapies, many will cling to the perceived or, perhaps, very real security of more rather than less antiretroviral therapy. Current triple-drug regimens are far from burdensome, toxic, or otherwise so different from DTG+3TC that patients will notice. An exception to this thinking, of course, is the potential differential effect of a two- versus three-drug regimen on the pocketbooks of insurers. Less medicine could mean lower costs and that may be an attractive attribute for bottom-line watchers, including the health care administrators in nations that are so advanced as to provide universal access to medications to their populace. So, we end the year knowing DTG+3TC as initial therapy works. Will HIV providers and patients be motivated to adopt it without being forced to? That is a question for another year.
- DTG in Early Pregnancy and Possible Neural Tube Defects
A much less welcomed DTG-related surprise came with a warning that the electrons carrying it could not deliver to inboxes around the globe fast enough. In early 2018, a research team conducting the Tsepamo Study, a large observational study of pregnant women in Botswana, found an association between early maternal DTG exposure and defects of the neural tube of infants.2, 3
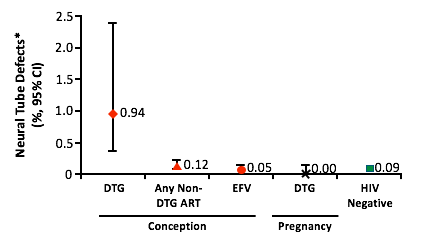
The many details of this story are important. First, Tsepamo is NIH-funded, is led by a team from the Harvard AIDS Partnership in Botswana, and is designed to look specifically for associations between HIV status, antiretroviral exposure during pregnancy, and infant neural tube defects. Second, in this amazing project, trained midwives at eight different hospitals physically exam infants born to women with and without HIV infection for signs of neural tube defects. Third, neural tube defects due to drug exposure occur during the first 28 days or so from conception. Fourth, in May 2016, Botswana shifted from TDF/3TC/efavirenz (EFV) to TDF/FTC + DTG as first-line antiretroviral therapy. Fifth, folic acid supplementation during pregnancy is not standard in Botswana. Lastly, the research team was asked by the World Health Organization (WHO) to look at the accumulated data to determine if a risk of neural tube defects in infants born to mothers exposed to EFV could be detected.
This initial unplanned analysis of 88,755 examined live births did not show a link between EFV and neural tube issues; however, comparativelythere was a higher prevalence of neural tube defects in infants whose mothers were taking DTG at the time of conception.2Of the 86 infants with neural tube defects identified, 4 were born to the 426 mothers taking DTG at conception – a rate of 0.94%. In comparison, neural tube defects were rarer in the 11,300 infants whose mothers were exposed at conception to any non-DTG antiretroviral (0.12%), the 66,057 infants whose mothers were HIV-uninfected (0.09%), and even the 2,812 infants whose mothers were exposed to DTG later in pregnancy (0.0%).
In a subsequent update with an increase in the number of women exposed to DTG at conception to 596, no additional cases of infant neural tube defects were identified, dropping the prevalence rate in this group to 0.67%.3 The next analysis will be conducted in March 2019.
The Bottom Line: The suggestion that an antiretroviral taken by a mother can harm her infant is gravely concerning under any circumstance – recall that it was birth defects due to a medication that helped usher in the current rigorous US drug approval system. In the case of DTG, the possibility that it could increase the risk of neural tube defects threatens to undercut a promising approach to HIV therapy that is EFV- and protease inhibitor-free. DTG is much better tolerated than these older regimens, and recent results from the DolPHIN-1 trial show that this very integrase inhibitor produced more rapid declines in the viral load of pregnant women, with larger proportions having undetectable levels of viremia at the critical time of birth than women taking EFV.4It is important to underscore that the Tsepamo Study did not find an association between neural tube defects and maternal exposure to DTG later in pregnancy.
After the study findings were announced, women of the AFROCAB, an advocacy network of representatives across Africa, issued a communique urging caution and a resistance to conclusion-jumping.5They call for a measured and shared approach to decision-making that includes women and point out that the issue of DTG and birth defects touches many women, not only those who are or are planning to become pregnant. Importantly, better access to affordable contraception was a centerpiece of the recommendations from this group.
Perinatal guidelines have been updated to reflect the data, which remain preliminary. Generally, these recommendations are consistent and logical. But, as the AFROCAB advocates stressed, decisions regarding the use of DTG in women who are or could become pregnant must be made not through the consensus of guideline committees but by a woman and her health care provider.
- Integrase Inhibitor Weight Gain
Well tolerated, with a high barrier to resistance, and potent as all-get-out, integrase inhibitors have revolutionized HIV treatment – as a glance at HIV treatment guidelines over the past few years makes it clear. But they are not without their downsides. Neuro-psych effects are rare but appears to be a family curse. And, there is the issue of neural tube defects, at least for DTG, as detailed above. Yet, these drawbacks have yet to significantly limit the use of these drugs. This past year, however, a crescendo of data suggest that integrase inhibitors may promote gains in weight beyond that seen with other antiretrovirals, and that could become a threat. Besides being unwelcomed by many patients, excess weight carries health risks. Additionally, in the US, most starting HIV therapy are already at least overweight – especially in the South.
The ‘do they or don’t they’ question of integrase inhibitors and weight gain has been looked at in a few studies presented/published this year – almost all retrospective. One of the first and typical of the rest is a report from Vanderbilt, located in the heart of the US obesity belt.4There 136 patients who switched from TDF/FTC/EFV to an integrase inhibitor experienced a mean gain of 2.9 kg, which was significantly greater than a mean 0.7 kg gain in the 34 changing to a protease inhibitor, and a 0.9 kg average gain among the 325 remaining on TDF/FTC/EFV.6
Studies of treatment-naïve antiretroviral initiators provide a more level playing field to assess differential effects on weight. A retrospective analysis of 3,208 patients at Parkland Hospital in Dallas who started HIV therapy between 2009 and 2017 looked at the changes in their body mass indexes (BMI) over time.7 Again, integrase inhibitor therapy (presumably, mostly RAL) was associated with greater BMI increases than treatment with other antiretroviral classes but, this was more likely to be seen in Black and Hispanic patients than White patients. Further, both integrase inhibitors and protease inhibitors led to higher BMI gains in women than in men.
At the Co-Morbidities Conference in late 2018, the NA-ACCORD cohort ‘weighed’ in.8Over 21,800 individuals in the US and Canada who started HIV therapy between 2007 to 2015 – almost 4,100 began with an integrase inhibitor in the mix (51% RAL, 37% elvitegravir [EVG], and 12% DTG) – were included in analyses that looked at actual and predicted changes in weight over time. Overall, weight increased (quite a bit) with all regimens, regardless of composition. Those initiating a non-nucleoside saw a median 4.1 kg gain over the first 5 years of treatment, compared to 5.0 kg with a protease inhibitor, and 5.8 kg with an integrase inhibitor. In pairwise comparisons treatment with either a protease inhibitor or an integrase inhibitor was associated with significantly greater increases in 5-year weight gain compared to treatment with a non-nucleoside, with no discernable difference between integrase and protease inhibitors. When examining 2-year changes in weight among the integrase inhibitors, EVG was linked to less average weight gain (3.4 kg) than RAL (5.4 kg) and DTG (5.6 kg). In models predicting changes in weight over time, integrase inhibitors are projected to produce the greatest increase in weight, with DTG having a great effect than other drugs of this class.
The observed NA-ACCORD data are congruent with the findings of a body composition sub-study of US AIDS Clinical Trials Group (ACTG) study A5257, in which treatment-naïve patients were randomized to start TDF/FTC plus either RAL versus atazanavir/ritonavir versus darunavir/ritonavir.9Here, no major differences in regional fat, lean mass, or BMI over 96-weeks of follow-up were observed in those assigned RAL and those assigned to the protease inhibitor arms. In this study, there was no non-nucleoside arm.
The Bottom Line: During the first half of the HIV epidemic, when being gaunt was stigmatizing, most patients appreciated the gain in weight that accompanied the initiation of HIV therapy. Putting on pounds was, and to some extent remains, a welcome indicator of the effectiveness of medication and a reassuring sign of a return to health. But, once potent therapies came along to stomp viral replication and stop opportunistic conditions, obesity replaced wasting as the major body issue for people living with HIV infection. Out with growth hormone and in with food pyramids and low-carb diets! Linked to diabetes mellitus, cardiovascular disease, and even frailty, excessive weight in people living with HIV has become a target for intervention. So, a love handle-expanding antiretroviral is not what the world needs now.
Collectively, the studies described above sketch a picture maybe of less weight increase with non-nucleosides compared to other antiretroviral classes. However, this is not an easy thing to study. The majority of non-nucleoside use is with EFV, a medication taken on an empty stomach. Switching from a regimen that has food restrictions to one that does not, could conceivably, have an impact on caloric intake and, therefore, weight. The same effect could be operative in the treatment-naïve – which may account for the finding in some studies of little difference between protease and integrase inhibitors.
However, some of the reports do signal a role for integrase inhibitors in relatively greater weight increases over time, perhaps, preferentially in certain groups. It is hard to shake a label. Although true evidence of a direct effect of protease inhibitors on increases in visceral fat does not exist, many still believe in Crix-belly and protease paunch. The same can happen with integrase inhibitors if well-conducted studies are not conducted and presented clearly. At a minimum, the differential effects of integrase inhibitors on weight can be addressed by the grand head-to-head naïve and switch trials of these agents – including studies that have already been conducted. Databases need to be dusted-off and those carefully conducted weights obtained at study visits compared across treatment arms. Future studies should include weigh change as an outcome. Only by baring all will we know what is truly happening and then why.
- PrEP and the Decline in New HIV Diagnoses
Fact #1: New diagnoses of HIV infection dropped by almost 20% from 2008 to 2015.
Fact #2: PrEP uptake increased dramatically during the same period.
Cause-and-effect or happy accident? A team from Emory and the neighboring Centers for Disease Control and Prevention (CDC) think it is the former.10Using the National HIV Surveillance System and a US prescription database, this team ranked US states and Washington, D.C. by their estimated annual change in HIV diagnoses, as well as their use of PrEP by those with an indication for PrEP. Then they placed each into quintiles to look at associations between PrEP use and new HIV case reports. Lastly, to isolate the effect of PrEP on new diagnoses from treatment as prevention, data on viral suppression available from 38 jurisdictions were also evaluated.
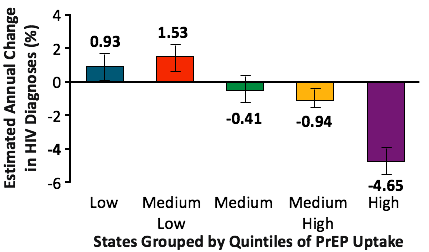
In the lowest PrEP uptake quintile, the estimated annual percent change in HIV diagnoses increased by 0.9%. In stark contrast, among the ten jurisdictions with the greatest uptake of indicated PrEP, the annual percent change in HIV diagnoses dropped 4.7%.For the quintiles between these extremes, the change in new diagnoses followed this trend. Controlling for viral suppression rates did not significantly alter these findings.
Interestingly, in 2016, the rate of PrEP use was more than three-times greater in the highest quintile of PrEP use than lowest quintile (110/1000 versus 35/1000).
The Bottom Line: Back in the days before potent combination therapy, long before any talk of broadly neutralizing antibodies, kick-and-kill, or long acting injectable, news of the discovery of a pill that could prevent a person from being infected with HIV would have been second only to a cure as a cause for mass euphoria. Maybe it is because of these other advances that PrEP’s reception has been so muted.
These data support a major role of PrEP, along with treatment as prevention, as a driver of the remarkable declines in new diagnoses of HIV. While the investigators nicely show where PrEP use is expanding and where it has not, neglected is who is being left out. PrEP is most available to white men who have sex with men (MSM). The rate of HIV diagnoses increased in MSM of color during the same period of time covered in this analysis– and, of course, African-Americans and Latinx are also less likely to be on PrEP.
Having PrEP be available and acceptable to all at-risk should not be this difficult. On the supply-side, many are breathlessly preaching PrEP to all potential prescribers who will listen – often to silence or indifference. Demand-side, things are complicated. Certainly, a pill-a-day prevention strategy has limitations – just ask anyone on lifelong antihypertensive or diabetes medication, or women who take oral contraception. Survey data show that some are not into a daily pill. But, the costs associated with being on PrEP and an under-appreciated risk are more powerful forces.11Again, lack of money and lack of knowledge are obstacles to effective means to protect health and well-being for people living in the US. The failure to have PrEP to be more widespread smacks of moral lethargy, complacency, and a bankruptcy of leadership – especially after this important piece of evidence. How about we Make America Healthy Again.
- Prévenir – On-Demand Prevention in France with Global Implications
As per above, PrEP works, but is daily dosing really necessary? For many, the dictum of a pill-a-day keeps the HIV away, is just fine. Yet, some have a harder time taking medications daily. A few years ago, the iPERGAY study planted the idea of an alternative PrEP strategy, demonstrating high-level protection from HIV infection with ‘on-demand’ PrEP – dosing of TDF/FTC before sex (two tablets 2 to 24 hours before) and then after (one tablet 24 hours and 48 hours after first dose). That trial was relatively small at 361 participants enrolled.12
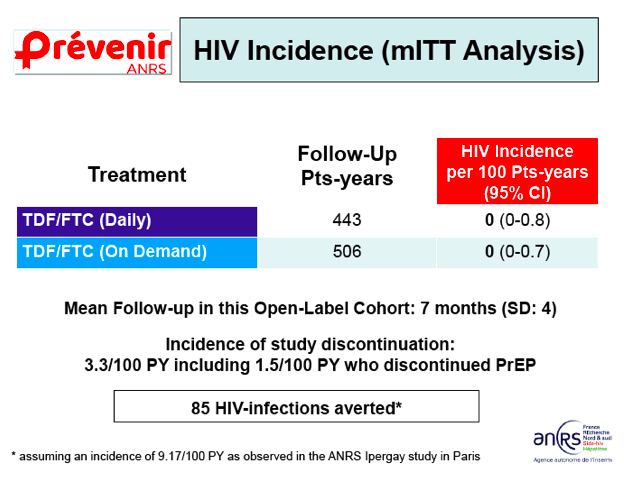
At the International AIDS Society meeting this summer, the same investigators presented interim findings from Prévenir, an on-going study that will enroll 3,000 HIV-uninfected people with an indication for PrEP in the Paris region.13Creatively, participants get to choose which approach they want, daily dosing or the IPREGAY dosing, rather than be randomized; plus, switching between strategies is allowed. The study primarily aims to show that with either strategy being available, a 15% or more dent in new diagnoses of HIV in the greater Paris region.
Preliminary data including the first 1,628 participants enrolled (98% MSM) were presented. On-demand PrEP was selected by 54% and, interestingly, at baseline these participants reported less risky behaviors relative to those who opted for daily PrEP. During a mean 7 months of follow-up, people generally stuck with their selected option and were pretty good at using it as directed. There was plenty of sex including condom-less sex, although more so in the daily PrEP group. So far, no cases of HIV seroconversion have occurred with either daily or on-demand PrEP and no participant has stopped PrEP due to an adverse event. Extrapolating from the serocoversion rate the iPERGAY trial, the investigators calculate 85 cases of HIV infection have been averted in Prévenir to date.
The Bottom Line: On-demand PrEP, like Jerry Lewis and Mickey Rourke, may be big in France, but in the US, not so much. Sure, we have our hands full just getting many primary care providers to know what PrEP is, and the data supporting on-demand PrEP, thus far, have been limited, but, with the interim findings from Prévenir, we need to look at how to use this strategy to help get PrEP to more who can benefit from it. This means departing from our tendency to protocol-ized PrEP. Fear-based dogma that necessitate strict adherence to every three-month clinic visits and over-aggressive lab testing are creating barriers to PrEP. Prévenir importantly allows for individuals to customize their PrEP and we can learn a lesson from this thinking. Clinics that provide PrEP can also develop ways to de-medicalize and streamline the process. For example, for the busy student who has demonstrated reasonable adherence to PrEP and evaluations, it is not sacrilegious to consider providing him four or even six PrEP refills along with instructions to get HIV and STI tested at the local health department in between clinic visits. Results can be sent through an electronic medical records portal or other secure method. The kind of reason and creativity that has been demonstrated by the Prévenir team can help us in getting PrEP to the next level in the US.
- Bictegravir has Arrived
I had to double check that it really was in 2018 that bictegravir was FDA approved. On February 7th, the approval of the fixed dose forumulation of bictegravir (BIC) with tenofovir alafenamide fumurate (TAF) and FTC was announced – but, like your teenage child, it seems like it has been around much longer.
Over these past months, this medication has come as close as any ever to being the default antiretroviral therapy– something to be used unless a really good reason dictates otherwise. Its main attraction is not so much about what it does, but for what it doesn’t: BIC+FTC+TAF does not contain a pharmacological booster, need HLA-B*5701 screening, have food requirements, or require avoidance in those with active HBV infection or certain CD4+ or HIV RNA parameters. Drug interaction-wise, it plays well with most meds that the average American might be prescribed. Plus, it is a single small pill. Clinical trial data show it is just as good as abacavir (ABC)+3TC+DTG, but with less hassle, and less nausea.14Compared to TAF/FTC + DTG, it is practically indistinguishable but a single tablet.15
The downsides of this combo are few but include the potential for the seemingly pan-integrase inhibitor neuropsychological issues, the potential for it to have similar neural tube defect risks as DTG (plus the general unknown safety of TAF in pregnancy), the potential for excess weight gain compared to other regimens, and the potential for tad more drug interactions than DTG. A lot of “potentials” without a lot of data. And, that is the major gripe about this medication – that it is new and, therefore, does not have a long track record.
The Bottom Line:In many ways, BIC+FTC+TAF, is the HIV med we have been waiting for but, it says a lot about where we have come to in HIV therapeutics that even this powerful and well-tolerated medication, and to some extent its two-pill sib DTG + TAF/FTC, have not been met with more fanfare. It could be that its immediate predecessors were pretty okay. Most patients taking single tablet regimens such as ABC+3TC+DTG or cobicistat-boosted EVG+TAF+FTC, and even many boosted protease inhibitor-based regimens, for the most part, have no complaints. While theoretically, getting off the ABC or the booster may offer benefits, they are not always so obvious to the people doing the medication-taking.
Which returns us to the idea of what will it take to lodge a triple-drug therapy like this one from HIV therapy pole position? Perhaps it will be a pill like DTG+3TC that contains less medicine, but if not, then it might will take something that isn’t a pill. Long acting injectable agents are the future (whether we know it and accept it, or not). Until then, on the multiple-choice test of what antiretroviral to use in most patients starting HIV medications, if you guess of BIC+FTC+TAF, chances are you will be right.
- Abacavir, Platelets, and a Biological Mechanism for Risk of CVD
Debates about the role ABC plays in the development of cardiovascular disease in persons living with HIV infection have raged for over a decade and a kind of stalemate has been achieved between those who are and are not convinced of a link. What has been missing has been a plausible biological mechanism by which ABC does what some fear it can to increase the risk of heart attacks and stroke. At the 2018 Conference on Retroviruses and Opportunistic Infections (CROI), data were presented that may finally solve this how-done-it.
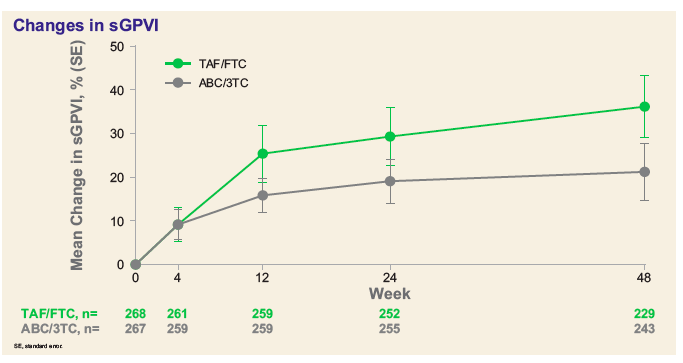
A collaborative from the United Kingdom (UK) looked at participants in a Gilead-sponsored trial that randomized 556 patients with suppressed viremia on an ABC+3TC containing regimen to either continue this regimen or switch their nucleosides to TAF+FTC.16The UK investigators performed intensive platelet function studies in a subset of 61 participants (29 who switched to TAF+FTC and 32 who remained on ABC+3TC) at one of four sites in Dublin or London (fresh blood was needed for the platelet testing). Platelet aggregation provoked by different agonists were assessed with significant inter-arm differences observed, indicating a greater tendency for platelets from those remaining on ABC+3TC to aggregate. Also examined was glycoprotein VI (GPVI), a collagen receptor on platelets that can trigger activation. Both platelet surface expression of GPVI in the 61 substudy participants and levels of soluble GPIV in the total cohort of 556 were looked at. It is a complicated bit of cell biology but low levels of soluble GPIV are thought to be associated with coronary artery events, and, thus not good. Those who switched from ABC+3TC to TAF+FTC saw an increase in expression and mean soluble GPVI over time – suggesting “an inherent defect in participants on ABC” that was reversible with switch.
The Bottom Line:The presented data on platelet activity on and off ABC may be the most convincing we will ever see implicating this drug in cardiovascular disease. Other studies, including one from another UK team also presented at CROI,17also hone in on ABC and its effects on the complex biology of the platelet. It should be noted that both UK studies were funded by Gilead, presumably through unrestricted grants, have yet to be published, and, generally, involve small numbers of participants. But, industry-independent clinical cohorts with large sample sizes have continued to publish associations between exposure to ABC and cardiovascular disease. At this same CROI, the venerable Swiss Cohort Study, for instance, found an independent association between treatment with ABC and non-calcified coronary plaque.18
With the dawn of DTG+3TC as well as TAF, the sun may be fading on ABC. Tolerance for the shadow of doubt cast on a medication is proportional to its usefulness. As the treatment of HIV infection has genuinely evolved to fitter agents, many of our older medications, including ABC, are becoming less relevant. Few patients need to be on ABC and there are alternatives that, on par, are probably better tolerated and safer.
- African-American Men and HIV Treatment Outcomes
The HIV pandemic, like the virus itself, is opportunistic and parasitic. AIDS is a disease of poverty, disruption, stigma, and chaos. Where ever the social fabric is torn, the infection enters and occupies. Wherever communities struggle, HIV thrives. Here in the US, where I sit, it is no different. We can applaud the advances described in this Top 10 and elsewhere, marvel at how far we have come and at a future bright with the possibility. But, the gaps between the haves and have-nots stare at us, and just as they do in the society we live, they force us to look back and acknowledge that our successes are hollow if not equally shared. For African-American men, the risk of acquiring HIV has been increasing at the same time the risk for white men has declined. Once infected, African-American men hit lower numbers at each step of the HIV Cascade of Care, compared to white MSM.19
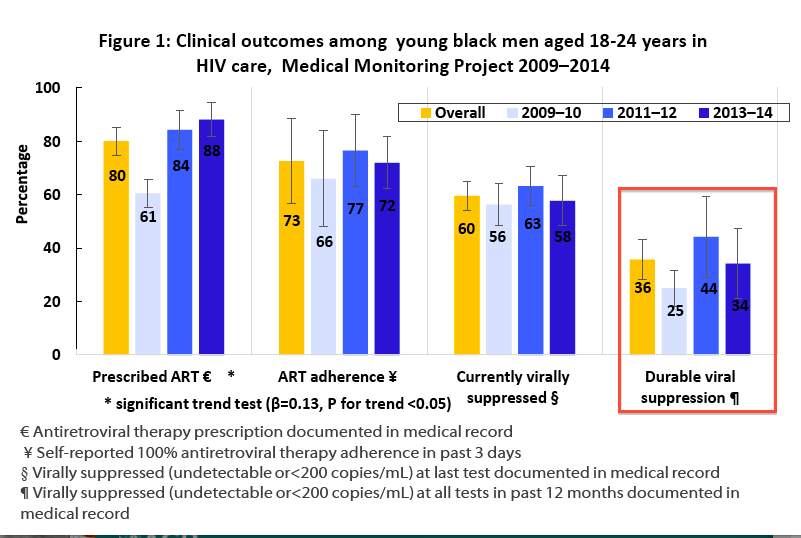
Early in 2018, the US Centers for Disease Control and Prevention (CDC) early in 2018 detailed the clinical outcomes of young African-American men in HIV care from 2009-2014 using data from the Medical Monitoring Project, a dataset representative of the adult HIV-infected population in the US.20During the study period, antiretroviral prescriptions generally increased from 61% to 88% among Black men. However, self-reported adherence to HIV therapy remained unchanged at around 70%. Similarly, there were no significant temporal changes in the rate of viral suppression at last visit (~60%) or more durable viral suppression over the prior 12 months (~36%).
A number of factors were associated with poor clinical outcomes including homelessness, depression, poverty, and smoking. Even taking a step back, before and analytics, the baseline characteristics of the men included were telling. Over half were living at or below the federal poverty level, 45% used illicit drugs, and 20% were depressed.
A similar story is told by an analysis of viral suppression among patients cared for at one of the 8 US Centers for AIDS Research Integrated Clinical Systems (CNICS) clinics.21Looking at viral suppression trends from 1997 to 2015, the investigators describe rates of HIV treatment success that increased markedly over this period but, unevenly with the odds of not having a suppressed viral load being significantly higher for African-Americans compared to Whites (odds ratio = 1.68 – 95% CI:1.57-1.80).
The Bottom Line: Many of us are called to HIV care by the confluence of medicine, public health, and social justice that a response to this epidemic entails. The CDC and CNICS data show us that disparities in the reach of advances in HIV care remain hard-baked into the system. Both papers call for tailored interventions that address adherence and social determinants of health. What this looks like is unclear and missing is a deep look at why outcome gaps are petrified – enduring over time as all boats rise (see figure below from the CNICS paper).

Most who consider such determinants, even those considered social, design their interventions for the individual – aiming to encourage health-seeking behaviors or inoculate against toxic structural forces. What about interventions that deal with racism and disenfranchisement, incarceration and the injustice of our justice system, underfunded basic education and uneven economic playing fields? What do those interventions look like? Will they come from well-intentioned social scientists with conceptual models and 5-year grants, or through a reckoning with a status quo that is fiercely defended while simultaneously literally killing people? HIV often reveals what ails a society, be it war, migration, or infanticide. Here in the states, of course, it is no different. When we fix how our society treats people of color and the poor and the marginalized, we will fix shameful disparities in the treatment of HIV. The biomedical and behavioral can be powerful but advances in policy are what we desperately need.
- The Long-Acting Bandwagon, Hop On!
Word is getting out that, barring some major setback, long-acting injectable antiretrovirals will soon be coming our way. Patients have come to clinic asking about it. Others muse that they wish there were shots instead of pills and are heartened when learning that there might be in the near future.
The LATTE-2 trial has continued to show the ability of cabotegravir (CAB) and RPV to maintain viral suppression and these will be the first injectable antiretrovirals to hit the market. But they will not be alone forever as others are working to get in on the long-acting game.Some notable agents under development include MK8591 (EFdA) is non-nucleoside reverse transcriptase translocation inhibitor (NNRTTI) – note the extra T – that has in vitro activity against HIV- and HIV-2, including multidrug resistant strains.22The active metabolite of this compound has been shown to have a half-life on the order of 120 hours, leading to options for oral dosing weekly, and perhaps much less frequently, if injected. Another interesting drug with long-acting potential is GS-9131, a nucleoside reverse transcriptase inhibitor, also is potent in vitro and has activity against resistant HIV.23Some are thinking beyond pills and needles to implants, tearing a page from the contraception playbook. Some preliminary work on a TAF implant was recently presented, fanning interest in this for prevention and treatment.24
The Bottom Line: Channeling Arthur C. Clarke and that guy in the Big Short who bet it all on the crash of 2008, I predict the future will be long-acting. It won’t be right away but, it will be. There are lots of reasons to think otherwise. Pills for HIV nowadays are small and amazingly well-tolerated. Shots hurt and there are plenty of needle-phobes. Plus, at first, injections will have to be given by someone with a white coat, rather than self-administered. Moreover, long-acting injectables go against the grain of some other conditions, like Multiple Sclerosis, where there is a desire for oral meds to take the place of injections. Then, there are the pregnancy issues.
Despite these challenges, some of which will be engineered out, I believe that freedom from a daily reminder of HIV infection is a powerful and liberating motivation for patients who wish to be like everyone else but can’t because of residual grains of virus slumbering in their cells. The longer the span between doses, the more these folks will want it. PrEP will follow or maybe even lead and further normalize shots to cover all HIV bases. This is a long-range vision. Shorter-term, we will need compatible agents to give together. This will mean a future in which companies play nice with one another.
- Designing HIV Resistant People is not a Good Thing
As unsavory as it is to most of us, the claim by Dr. He Jiankui that he was able to use Crispr-Cas9 technology to alter the genes of at least twins so that they were born with their CD4 cells not expressing the CCR5 co-receptor that HIV uses to gain cellular entry, is big news. First, there is the technical feat, which if verified is landmark. If this actually happened, Dr. He was able to edit the genes of two embryos so that all their cells are changed. This means that the genes they pass on to their own children will also be the edited versions. Apparently, only one of the twins had both copies of theCCR5 genes. For the other, only one copy of this gene was deleted and therefore CCR5 will still be expressed by some of her CD4 cells. More significant are the ethical aspects, which have gotten most of the attention. The safety of editing genes is not known and there are legitimate concerns that inadvertently other genes that are consequential to health could also have been altered or removed. Even if not, CCR5 deletion is associated with some negatives including more severe disease when infected with West Nile Virus, Japanese Encephalitis, and perhaps other infections. Further, these infants never consented to have their DNA altered by Dr. He, raising serious issues about the right to be born with the genes you are dealt, especially if there was nothing obviously wrong with them.
What is sometimes missed in the swirling ethical debate this has provoked, is the rationale for making these infants HIV-proof. It is reported that their father is HIV-infected. That Dr. He chose to perform his experiment on children whose father is HIV-positive signals that he somehow poses a risk to them, which, of course is preposterous. The genes of these girls have been manipulated to protect them from a menace that does not exist; any future risk of HIV infection they face can be better and more safely be addressed by standard HIV prevention methods.Certainly, Dr. He knows this. So, why did he do it, because he could.
The Bottom Line:In addition to the outrage over editing the genes of healthy embryos, which is illegal in the US and may carry significant risk to these children and their offspring, people living with HIV and their allies should strongly oppose Dr. He and condemn his cloaking of this work with the nobility of combating HIV. The noble cause of preventing HIV cannot be used to justify scientific recklessness. If it is true this has been done, and that a Chinese HIV advocacy group helped him recruit the parents, the worldwide HIV community must add their voice and condemn Dr. He and this organization.
- Cahn P, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naïve adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2018 Nov 9. Epub ahead of print
- Zash R, et al. N Engl J Med. Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. 2018 Sep 6;379(10):979-981
- Zash R, et al. Surveillance for neural tube defects following antiretroviral exposure from conception.22nd International AIDS Conference (AIDS 2018), Amsterdam, 2018.
- Orrell C, et al. DolPHIN-1: randomised controlled trial of dolutegravir (DTG)- versus efavirenz (EFV)-based therapy in mothers initiating antiretroviral treatment in late pregnancy. 22nd International AIDS Conference (AIDS 2018), Amsterdam, 2018.
- http://www.afrocab.info
- Norwood J, et al. Weight Gain in Persons with HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor–Based Regimens. J Acquir Immune Defic Syndr 2017;76:527–531
- Bedimo R, et al. Integrase Inhibitors-based HAART is Associated with Greater BMI Gains in Blacks and Hispanics.IDWeek, San Francisco, 2018
- Lake JE, et al. ART Initiation with Integrase Inhibitors is Associated with Greater Weight Gain than with PI- or NNRTI-Based ART in the US and Canada. 20thThe International Workshop on Comorbidities & Adverse Drug Reactions in HIV, New York, 2018
- McComsey G, et al, Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62(7):853–62
- Sullivan PS, et al. The impact of pre-exposure prophylaxis with TDF/FTC on HIV diagnoses, 2012-2016, United States. 22nd International AIDS Conference (AIDS 2018), Amsterdam, 2018.
- Scott HM, et al. Unmet PrEP demand and factors associated with PrEP interest among men who have sex with men in the United States. HIV Research for Prevention (HIVR4P), Madrid, 2018
- Molina JM, et al. On-Demand Preexposure Prophylaxis in Men at High Risk forHIV-1 Infection. N Engl J Med. 2015 Dec 3;373(23):2237-46.
- Molina J-M et al. Incidence of HIV-infection in the ANRS Prévenir study in Paris region with daily or on-demand PrEP with TDF/FTC. 22nd International AIDS Conference (AIDS 2018), Amsterdam, 2018.
- Wohl DA, et al. A Phase 3, Randomized, Controlled Clinical Trial of Bictegravir in a Fixed-Dose Combination, B/F/TAF, vs ABC/DTG/3TC in Treatment-Naïve Adults at Week 96. IDWeek, San Francisco, 2018
- Stellbrink H et al.Phase III randomized, controlled clinical trial of bictegravir coformulated with FTC/TAF in a fixed-dose combination (B/F/TAF) versus dolutegravir (DTG) + F/TAF in treatment naıve HIV-1 positive adults: Week 96 results. International Congress on Drug Therapy in HIV Infection (HIV Glasgow), Glasgow, 2018.
- Mallon P, et al. Platelet function upon switching to TAF vs continuing ABC: a randomized substudy. 25th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 2018.
- Taylor KA, et al. Comparative Impact of Antiretrovirals on Human Platelet Activation. 25th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 2018.
- Kovari H, et al. Antiretroviral Drugs Associated with Subclinical Coronary Artery Disease.25th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 2018.
- Rosenberg ES, et al. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: a modeling study. Lancet HIV 2014; 1: e112–18.
- Chowdury PP, Beer L, Shouse RL, Bradley H. Clinical outcomes of US young black men with HIV receiving medical care, 2009-2014. 25th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 2018.
- Nance RM, et al. HIVViral Suppression Trends Over Time Among HIV-Infected Patients Receiving Care in the United States, 1997 to 2015: A Cohort Study. Ann Intern Med. 2018 Sep 18;169(6):376-384
- Matthews RP, et al. Multiple dialy does of M-8591 as low as 0.25 mg are expected to suppress HIV. 25th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 2018.
- White KL, et al. GS-9131 is a novel NRTI with activity against NRTI-Resistant HIV-1. 24th Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, 2017.
- Gatto GJ, et al.Development of an end-user informed tenofovir alafenamide (TAF) implant for long-acting (LA)-HIV pre-exposure Prophylaxis (PrEP). HIV Research for Prevention (HIVR4P), Madrid, 2018