Dynamic profiling of the glioma kinome
Targeted kinase inhibitors represent a promising alternative to standard cytotoxic treatments for gliomas, including glioblastoma (GBM). But use of brain-penetrant drugs in combinations designed to overcome compensatory resistance mechanisms will likely be necessary to improve outcomes. Preclinical studies can aid identification of effective drug combinations.
Project summary
We investigate the molecular mechanisms of kinase inhibitor resistance using genetically-engineered mouse (GEM) models and human patient-derived xenografts (PDX).
In particular, we focus on the molecular mechanisms by which the kinome adapts to short- and long-term drug exposure.
Mechanistic studies use non-germline GEM (nGEM) and PDX culture systems to:
Examine how adaptive kinome changes contribute to kinase inhibitor resistance
Integrated proteomics and genomics analyses are critical for this work. These include kinome profiling using multiplex inhibitor beads and mass spectrometry (MIB-MS) and RNA-seq to examine drug-induced changes in the kinome and kinase transcriptome.
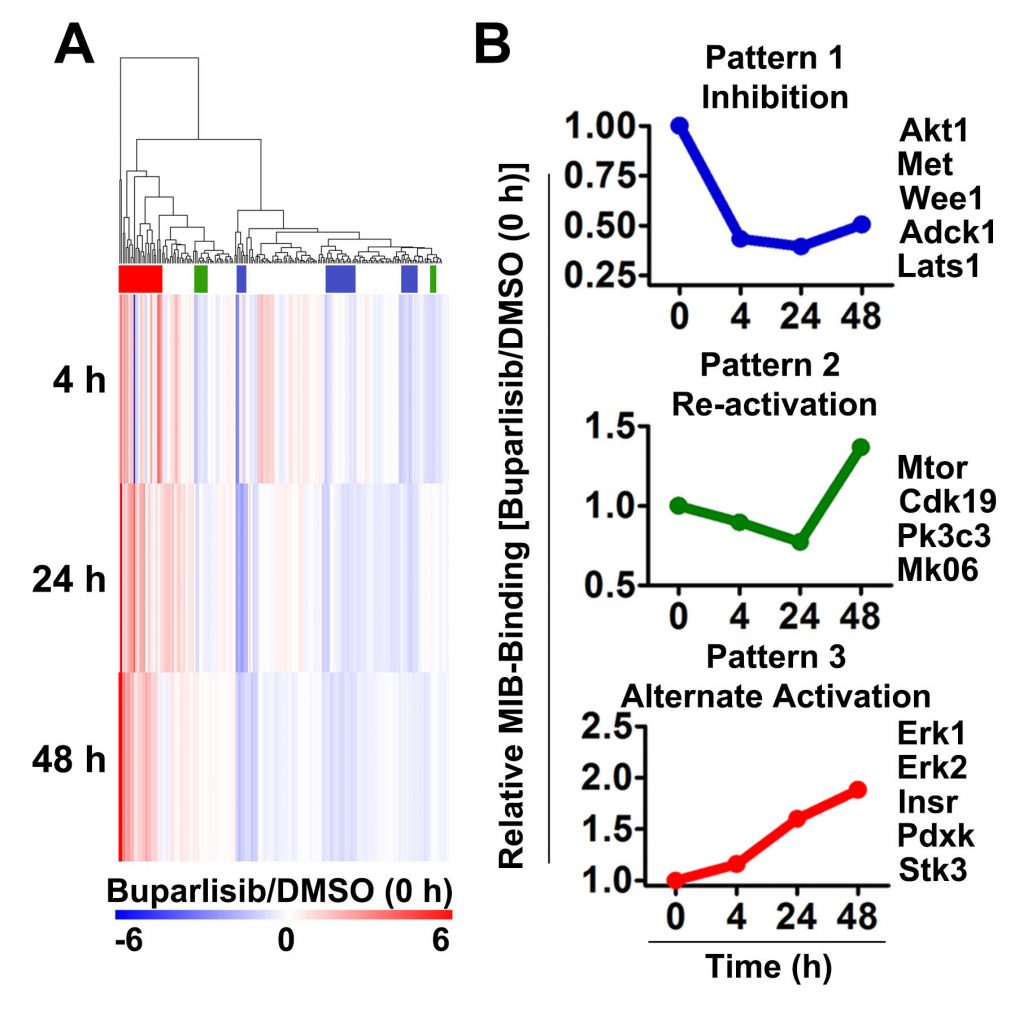
McNeill et al. Neuro-oncology 19(11):1469 2017
Define the kinome of human glioma model systems and tumors
We perform MIB-MS kinome and RNA-seq transcriptome profiling on established human glioma cell lines, PDX cultures, and human gliomas to identify kinase networks that may be useful for disease classification, improved molecular diagnostics, and development of predictive biomarkers for kinase inhibitor therapies.
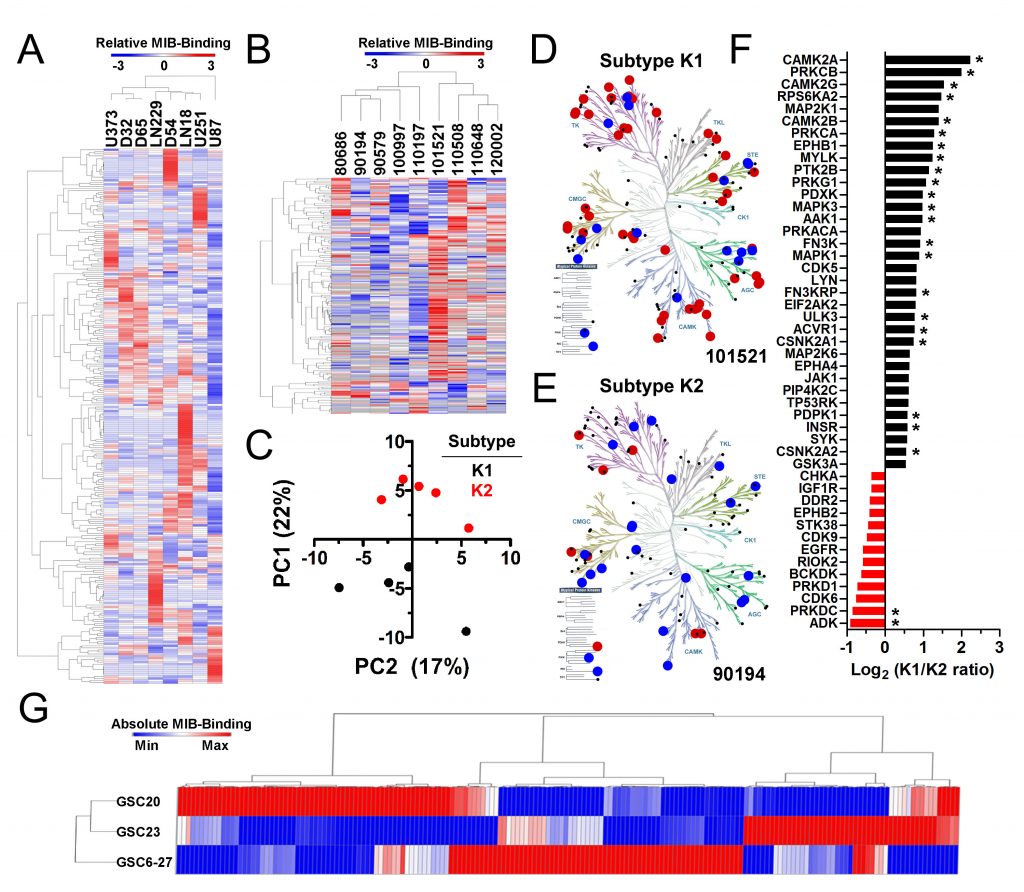
McNeill et al. Neuro-oncology 19(11):1469 2017
References
- McNeill RS, Stroobant EE, Smithberger E, Canoutas DA, Butler MK, Shelton AK, Patel SD, Limas JC, Skinner KR, Bash RE, Schmid RS, Miller CR. PIK3CA missense mutations promote glioblastoma pathogenesis, but do not enhance targeted PI3K inhibition. PLoS One. 13(7):e0200014 Jul 2018. PMID: 29975751 PMCID: PMC6033446
- Wu J, Frady LN, Bash RE, Cohen SM, Schorzman AN, Su YT, Irvin DM, Zamboni WC, Wang X, Frye SV, Ewend MG, Sulman EP, Gilbert MR, Earp HS, Miller CR. MerTK as a therapeutic target in glioblastoma. Neuro-oncology. 20(1):92-102 Jan 2018. PMID: 28605477 PMCID: PMC5761530
- McNeill RS, Canoutas DA, Stuhlmiller TJ, Dhruv HD, Irvin DM, Bash RE, Angus SP, Herring LE, Simon JM, Skinner KR, Limas JC, Chen X, Schmid RS, Siegel MB, Van Swearingen AED, Hadler MJ, Sulman EP, Sarkaria JN, Anders CK, Graves LM, Berens ME, Johnson GL, Miller CR. Combination therapy with potent PI3K and MAPK inhibitors overcomes adaptive kinome resistance to single agents in preclinical models of glioblastoma. Neuro-oncology. 19(11):1469-1480 Oct 2017. PMID: 28379424 PMCID: PMC5737415
- Van Swearingen AED, Sambade MJ, Siegel MB, Sud S, McNeill RS, Bevill SM, Chen X, Bash RE, Mounsey L, Golitz BT, Santos C, Deal A, Parker JS, Rashid N, Miller CR, Johnson GL, Anders CK. Combined kinase inhibitors of MEK1/2 and either PI3K or PDGFR are efficacious in intracranial triple-negative breast cancer. Neuro-oncology. 19(11):1481-1493 Oct 2017. PMID: 28486691 PMCID: PMC5737524
- McNeill RS, Vitucci M, Wu J, Miller CR. Contemporary murine models in preclinical astrocytoma drug development. Neuro-oncology. 17(1):12-28 Jan 2015. PMID: 25246428 PMCID: PMC4483055
- McNeill RS, Schmid RS, Bash RE, Vitucci M, White KK, Werneke AM, Constance BH, Huff B, Miller CR. Modeling astrocytoma pathogenesis in vitro and in vivo using cortical astrocytes or neural stem cells from conditional, genetically engineered mice. Journal of Visualized Experiments. 90:e51763 Aug 2014. PMID: 25146643
- Vitucci M, Karpinich NO, Bash RE, Werneke AM, Schmid RS, White KK, McNeill RS, Huff B, Wang S, Van Dyke T, Miller CR. Cooperativity between MAPK and PI3K signaling activation is required for glioblastoma pathogenesis. Neuro-oncology. 15(10):1317-1329 Oct 2013. PMID: 23814263 PMCID: PMC3779038