Research Projects
Exploiting inhibitory Siglecs to combat food allergy
Inhibitory Siglecs are present on key immunologic cells in food allergy, for example Siglec 2 (CD22) on B cells, and Siglec 3 (CD33) on mast cells and basophils. Our research aims are to target these Siglecs to delete peanut allergen-specific B cells and inhibit degranulation of mast cells and basophils via STALs (Siglec-engaging Tolerance-inducing Antigenic Liposomes). Specifically, we have demonstrated that Ara h 2 CD22-STALs prevent the production of Ara h 2-specific IgE in a mouse model of peanut allergy. More recently, we have generated data indicating tolerance of memory B cells following STALs treatment. Ongoing studies aim to build on promising preliminary data demonstrating the utility of Ara h 2 CD33-STALs to prevent basophil degranulation.
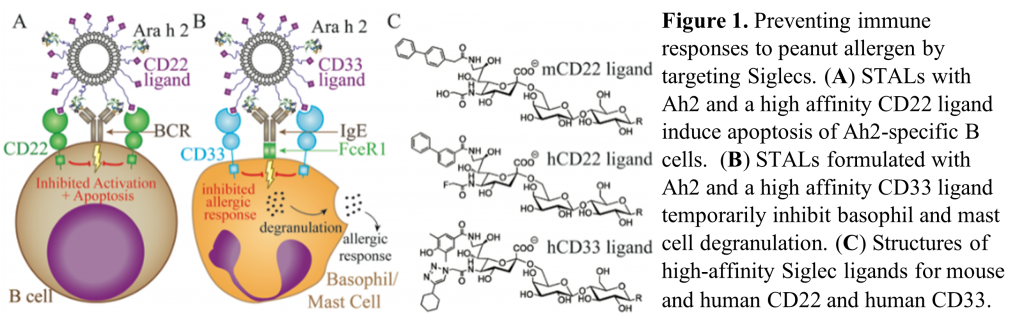
Publications:
- Exploiting CD22 on antigen-specific B cells to prevent allergy to the major peanut allergen Ara h 2 [Kelly A. Orgel, Wesley Burks, Mike Kulis | Journal of Allergy and Clinical Immunology | January 2017]
- Antigenic Liposomes for Generation of Disease-specific Antibodies [LaKeya Hardy, Johanna Smeekens, Mike Kulis | Journal of Visualized Experiments | October 2018]
Collaborator: Matthew Macauley, PhD – University of Alberta
Funding: DoD
Sensitization to peanut through the airway
Mechanisms underlying sensitization to foods are not well understood. Recently, the dual allergen exposure hypothesis has posited that non-oral routes of exposure, prior to oral exposure, lead to the development of food allergy. Cutaneous exposure has been the focus of many studies, but we are interested in the airway as a route of exposure. Specifically, we have developed a mouse model demonstrating sensitization to peanuts can occur through the airway using house dust extract as an adjuvant.
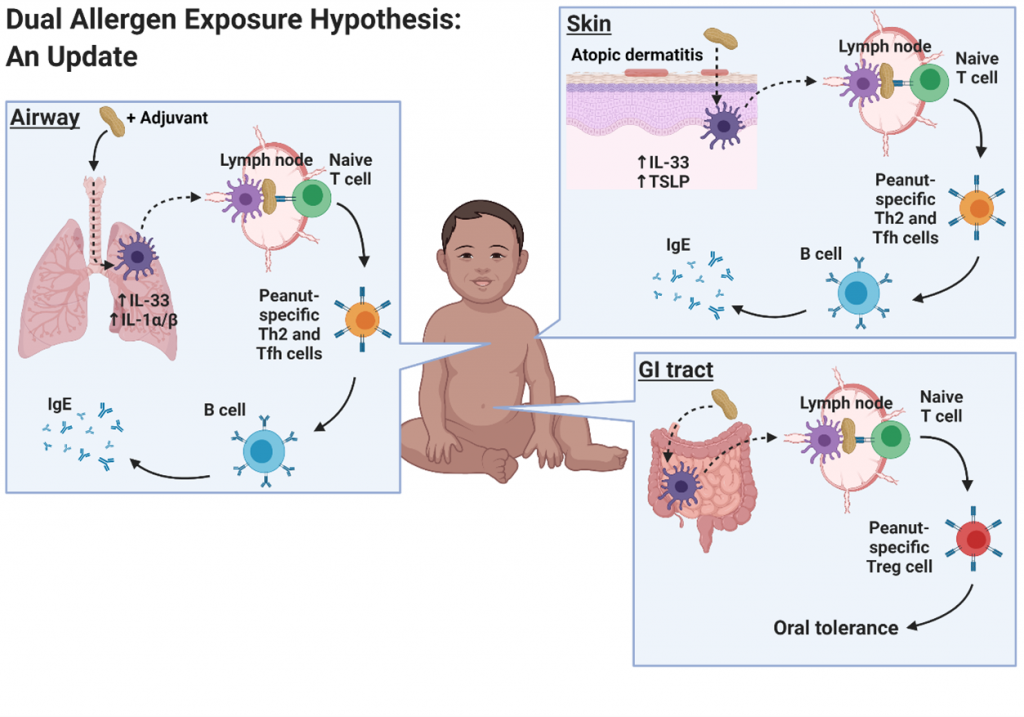
Publications:
- Indoor dust acts as an adjuvant to promote sensitization to peanuts through the airway [Johanna Smeekens, Mike Kulis, Tim Moran | Clinical & Experimental Allergy | August 2019]
- House dust promotes sensitization to peanuts through the airway [Johanna Smeekens, Mike Kulis, Tim Moran | Journal of Allergy and Clinical Immunology | February 2019]
Collaborator: Timothy Moran, MD, PhD – University of North Carolina
Funding: CEHS
A genetically-susceptible mouse model of peanut allergy
Over the past five years, we have tested several strains of mice from the UNC collection of Collaborative Cross mouse strains and identified one in particular, CC027/GeniUnc, that is susceptible to developing peanut allergy. Importantly, these mice can be orally sensitized to peanut, in the absence of a Th2-skewing adjuvant, and react upon oral challenge to peanut. We have identified differences in immunologic responses between CC027/GeniUnc and other strains of mice, including increased number of mast cells in the gut, decreased Tregs, and lower levels of fecal IgA. Furthermore, there are distinct gut microbiome differences associated with the development of peanut allergy, including lower levels of Akkermansia.
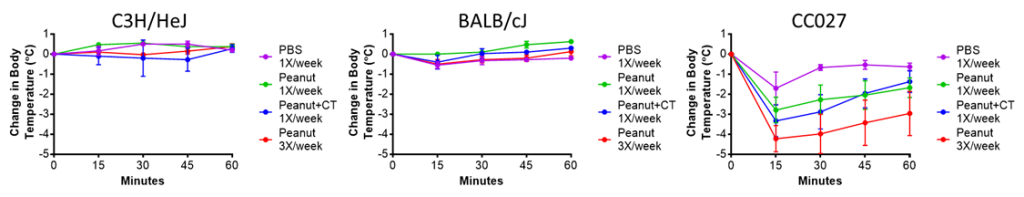
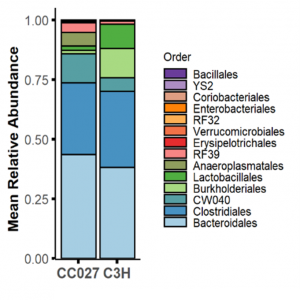
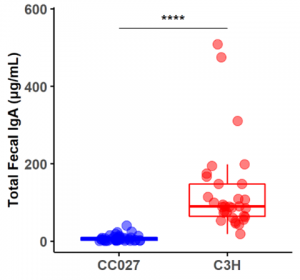
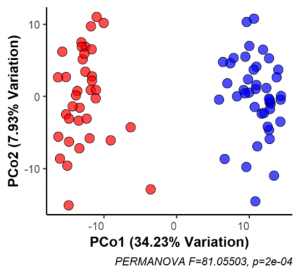
Publications:
- Genetic diversity between mouse strains allows identification of the CC027/GeniUnc strain as an orally reactive model of peanut allergy [Kelly Orgel, Johanna Smeekens, Ping Ye, Rishu Guo, Wesley Burks, Mike Kulis | Journal of Allergy and Clinical Immunology | October 2018]
- Fecal IgA, Antigen Absorption, and Gut Microbiome Composition Are Associated With Food Antigen Sensitization in Genetically Susceptible Mice [Johanna Smeekens, Andrew Hinton, Tim Moran, Janelle Kesselring, Erin Steinbach, Kelly Orgel, Wesley Burks, Mike Kulis | Frontiers in Immunology | January 2021]
- Model of Walnut Allergy in CC027/GeniUnc Mice Recapitulates Key Features of Human Disease [Johanna Smeekens, Kelly Orgel, Janelle Kesselring, Mike Kulis | Yale Journal of Biology and Medicine | December 2020]
Collaborators: Martin Ferris, PhD – University of North Carolina | Fernando Pardo-Manuel de Villena, PhD – University of North Carolina
Previous funding: NC TraCS TTSA award
CoFAR Central Biomarker Facility
The UNC FAI is home to the NIH-sponsored Consortium of Food Allergy Research (CoFAR) Central Biomarker Facility. In this role, we perform two major functions: conducting biomarker assays and acting as a repository for biological specimens generated from clinical trials. In particular, we are performing basophil activation tests (BATs) and serological measurements of food-specific antibodies for the ongoing OUTMATCH study. Within the repository, UNC houses serum, plasma, PBMCs, saliva, stool, and urine. We will also play a key role in the SUNBEAM birth cohort study quantifying food-specific antibodies and storing biological samples.
Current funding: CoFAR Biomarker