Michael Emanuele’s and Nicholas Brown’s Labs have published a new paper in PLOS Biology titled, “In silico APC/C substrate discovery reveals cell cycle-dependent degradation of UHRF1 and other chromatin regulators.”
This study shows that the cell cycle E3 ubiquitin ligase APC/C is a regulator of several chromatin regulatory proteins, including the multivalent epigenetic reader and writer UHRF1. Jennifer L. (Kernan) Franks, alumni graduate student of the Emanuele Lab is first author.
Abstract
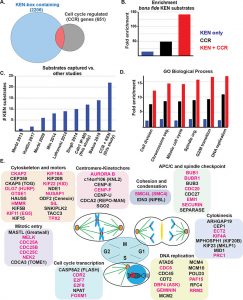
The anaphase-promoting complex/cyclosome (APC/C) is an E3 ubiquitin ligase and critical regulator of cell cycle progression. Despite its vital role, it has remained challenging to globally map APC/C substrates. By combining orthogonal features of known substrates, we predicted APC/C substrates in silico. This analysis identified many known substrates and suggested numerous candidates. Unexpectedly, chromatin regulatory proteins are enriched among putative substrates, and we show experimentally that several chromatin proteins bind APC/C, oscillate during the cell cycle, and are degraded following APC/C activation, consistent with being direct APC/C substrates. Additional analysis revealed detailed mechanisms of ubiquitylation for UHRF1, a key chromatin regulator involved in histone ubiquitylation and DNA methylation maintenance. Disrupting UHRF1 degradation at mitotic exit accelerates G1-phase cell cycle progression and perturbs global DNA methylation patterning in the genome. We conclude that APC/C coordinates crosstalk between cell cycle and chromatin regulatory proteins. This has potential consequences in normal cell physiology, where the chromatin environment changes depending on proliferative state, as well as in disease.
Read the paper published in PLOS Biology, Dec 11, 2020, https://doi.org/10.1371/journal.pbio.3000975
First author on the paper is Jennifer L. Franks. Besides Michael Emanuele and Nicholas Brown, other coauthors include Raquel C. Martinez-Chacin, Xianxi Wang, Rochelle L. Tiedemann, Thomas Bonacci, Rajarshi Choudhury, Derek L. Bolhuis, Taylor P. Enrico, Ryan D. Mouery, Jeffrey S. Damrauer, Feng Yan, Joseph S. Harrison, M. Ben Major, Katherine A. Hoadley, Aussie Suzuki, and Scott B. Rothbart.
