Defining the molecular pathway of Cerebral Cavernous Malformations (CCM) Pathogenesis.
Research Project by Asya Borikova
 Cerebral Cavernous Malformations (CCM) are dilated, hyperpermeable microvessels found primarily in the brain. The clinical manifestations of CCM are neurological deficits and stroke. Current treatment for the disease is surgery, however this caries a high risk to the patient and lesions can recur. CCM can be familial or sporadic.
Cerebral Cavernous Malformations (CCM) are dilated, hyperpermeable microvessels found primarily in the brain. The clinical manifestations of CCM are neurological deficits and stroke. Current treatment for the disease is surgery, however this caries a high risk to the patient and lesions can recur. CCM can be familial or sporadic.
Genetic analysis of familial cases has indicated that premature truncations or point mutations within three genes, ccm1, ccm2 or ccm3 are associated with pathogenesis. The Johnson lab showed that CCM1, 2 and 3 are scaffold-like adaptor proteins which regulate the stability and activity of the small GTPase RhoA. Loss of CCM1, 2 or 3 expression leads to increased RhoA stability and activity, resulting in increased activation of the RhoA effector Rho Kinase (ROCK) and increased phosphorylation of the ROCK target myosin light chain 2 (MLC2). MLC2 is an essential component of the actinomyosin cytoskeleton and the constitutive phosphorylation of MLC2 in CCM deficient endothelial cells leads to increased stress fiber formation, impaired motility and impaired organization of vascular-like lumens in vitro.
The molecular signals which lead to the hyperactivation of RhoA are currently unknown and their identification can lead to potential drug targets for the treatment of CCM. My research focuses on defining the molecular mechanism leading to RhoA activation in CCM deficient endothelial cells.
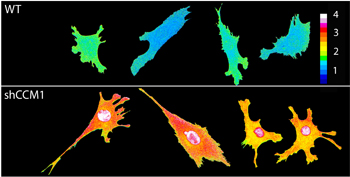
Figure legend: Endothelial cells were infected with a FRET-based RhoA biosensor (courtesy of Dr. Klaus Hahn) which emits a fluorescent signal proportional the number of RhoA molecules activated. Color heat bar represents RhoA activity. Loss of CCM1 expression leads to increased RhoA activity.