Research Interests
- Molecular mechanisms of membrane transport
Research Synopsis
Research efforts in this laboratory are directed toward an understanding of the molecular mechanism of membrane transport. The transport molecule of interest is the ATPase in the plasma membrane of Neurospora. The hydrolytic moiety of the ATPase has been identified as a polypeptide with a molecular mass of about 100,000 daltons, and the physiological role of the ATPase as an electrogenic proton pump has been established. This laboratory also has shown that the catalytic mechanism of the H+-ATPase involves transient phosphorylation and dephosphorylation of the beta-carboxyl group of an aspartic acid residue in the 100,000 dalton polypeptide chain, establishing the kinship of this ATPase with animal cell counterparts such as the Na+/K+-, H+/K+-, and Ca2+-ATPases of plasma membranes, and the Ca2+-ATPase of muscle sarcoplasmic reticulum. ATPase conformational changes during the catalytic cycle have been demonstrated as well. Procedures also have been developed for solubilizing and purifying active ATPase to near homogeneity in 100 milligram quantities, and for reconstituting the purified molecule in fully functional form into artificial phospholipid vesicles. Using this reconstituted system, this laboratory has recently demonstrated that the 100,000 dalton moiety alone is capable of efficient ATP-hydrolysis-driven proton translocation. The chemistry and physical properties of this transport molecule are currently being probed with the ultimate goal of a complete elucidation of its structure and molecular mechanism.
ATPase
Publications
- Scarborough , G.A. (2003) Rethinking the P-type ATPase problem. Trends Biochem Sci.28(11):581-4. Abstract
- Scarborough , G.A. (2002) Molecular mechanism of the P-type ATPases. J. Bioenerg. Biomemb. 3(4)4:235-250. Abstract
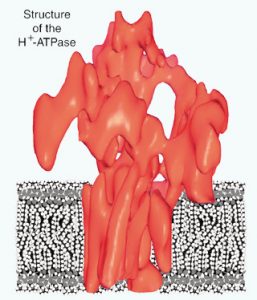
- Rhee, K.-H., Scarborough, G.A., and Henderson, R. (2002) Domain movements of plasma membrane H+-ATPase: 3D structures of two states by electron cryo-microscopy. EMBO J. 21(14):3582-3589. Abstract
- Scarborough GA. (2000) The plasma membrane proton-translocating ATPase. Cell Mol Life Sci. 5(6)7:871-883. Abstract
- Scarborough, G.A. (2000) Crystallization, Structure and Dynamics of the Proton-translocating P-type ATPase, J. Exp. Biol. 203(Pt.1):147-154. Abstract

