Led by graduate student Jeff DiBerto, the UNC School of Medicine lab of Bryan Roth teamed with scientists in China to publish detailed structures of the entire human opioid receptor family to guide the creation of more targeted pain medications.
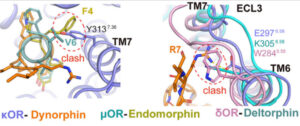
CHAPEL HILL, NC – In the continuing effort to improve upon opioid pain relievers, American and Chinese scientists used cryoEM technology to solve the detailed structures of the entire family of opioid receptors bound to their naturally occurring peptides. Subsequent structure-guided biochemical studies were then performed to better understand the mechanisms of peptide-receptor selectivity and signaling drugs.
This work, published in Cell, provides a comprehensive structural framework that should help drug developers rationally design safer drugs to relieve severe pain.
This work was spearheaded by the lab of Eric Xu, PhD, at the CAS Key Lab of Receptor Research in China, in collaboration with the lab of Bryan L. Roth, MD, PhD, at the UNC School of Medicine, where graduate student Jeff DiBerto led the pharmacological experiments to understand the receptors’ signaling mechanisms.
Opioid drugs relieve pain by mimicking a naturally occurring pain-relief function within our nervous symptoms. They are the best, strongest pain relievers we have. Unfortunately, they come with side effects, some severe such as numbness, addiction, and respiratory depression, leading to overdose deaths.
Scientists have been trying for many years to overcome the side-effect problem in various ways, all involving one or more of four opioid receptors to no avail. One way scientists continue to explore is the creation of peptide or peptide-inspired small molecule drugs.
Peptides are short chains of amino acids; think of them as short proteins. Certain naturally occurring, or endogenous, peptides bind to opioid receptors on the surface of cells to create an analgesic effect, also known as pain relief. Think of an analgesic like an anesthetic, except that analgesics do not “turn off” the nerves to numb the body or alter consciousness. So, the idea is to create a peptide drug that has a strong analgesic effect, without numbing nerves or altering consciousness or causing digestive, respiratory, or addiction issues.

“The problem in the field is we’ve lacked the molecular understanding of the interplay between opioid peptides and their receptors,” said Roth, co-senior author and the Michael Hooker Distinguished Professor of Pharmacology. “We’ve needed this understanding in order to try to rationally design potent and safe peptide or peptide-inspired drugs.”

“This collaboration revealed conserved, or shared, mechanisms of activation and recognition of all four opioid receptors, as well as differences in peptide recognition that can be exploited for creating subtype-selective drugs,” said DiBerto, first author and PhD candidate in the Roth lab. “We provide more needed information to keep pushing the field forward, to answer basic science questions we hadn’t been able to answer before now.”
The thrust behind such research led by Xu and Roth is to home in on the mechanistic reasons for pain relief potency without triggering the cellular mechanisms that lead to severe side effects and overdosing.
“We are attempting to build a better kind of opioid,” Roth says, “We’re never going to get there without these kind of basic molecular insights, wherein we can see why pain is relieved and why side effects occur.”
Co-first authors of the Cell paper are Yue Wang and Youwen Zhuang of the CAS Key Laboratory of Receptor Research and the State Key Laboratory of Drug Research at the Shanghai Institute of Materia Medica in the Chinese Academy of Sciences. Other authors are Edward Zhou and Karsten Melcher of the Van Andel Research Institute in Grand Rapids, MI, Gavin Schmitz and Manish Jain at the UNC School of Medicine, and Qingning Yuan, Weiyi Liu, and Yi Jiant at the CAS Key Laboratory.
The above is excerpted from the original article published on UNC Health and UNC School of Medicine Newsroom, by Mark Derewicz, January 12, 2023. Read the original article for more details on how they used cryogenic electron microscopy, or cryoEM, and a battery of biomechanistic experiments in cells to solve the detailed structures of endogenous peptides bound to all four opioid receptors.
Brian Roth and his lab are members of the UNC Pharmacology Department. Jeff DiBerto is a graduate student in the Pharmacology Graduate Program.
