Led by Juan Song, PhD, scientists at the UNC School of Medicine used optogenetic techniques to stimulate specific brain cells to increase production of neural stem cells and neurons relevant to memory and emotion processing in animal models.
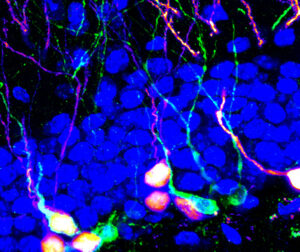
We humans lose mental acuity, an unfortunate side effect of aging. And for individuals with neurodegenerative conditions such as Alzheimer’s and Parkinson’s, the loss of cognitive function often accompanied by mood disorders such as anxiety is a harrowing experience. One way to push back against cognitive decline and anxiety would be to spur the creation of new neurons. For the first time, University of North Carolina School of Medicine scientists have targeted a specific kind of neuron in mice to increase the production of neural stem cells and spur on the creation of new adult neurons to affect behavior.
Targeting these cells, as reported in the journal Nature Neuroscience, modulated memory retrieval and altered anxiety-like behaviors in mice. Essentially, the UNC scientists boosted the electrical activity between cells in the hypothalamus and the hippocampus to create new neurons – an important process called neurogenesis.
“Targeting the hypothalamic neurons to enhance adult hippocampal neurogenesis will not only benefit brain functions,” said senior author Juan Song, PhD, associate professor of pharmacology, “but also holds the potential to treat cognitive and affective deficits associated with various brain disorders.” ~Juan Song, PhD, senior author

Most neurons we carry for life were created before we were born and get organized during early childhood. But such neurogenesis continues into adulthood and throughout life. In fact, one of the reasons for cognitive decline and anxiety, and even diseases such as Alzheimer’s, is the suspension of neurogenesis.
Impaired adult hippocampal neurogenesis correlates with many pathological states, such as aging, neurodegenerative diseases, and mental disorders. “Therefore,” Song added, “targeting the hypothalamic neurons to enhance adult hippocampal neurogenesis will not only benefit brain functions but also holds the potential to treat cognitive and affective deficits associated with various brain disorders.”
~ the above are excerpts from the full article of the same title by Mark Derewicz, published in UNC HealthCare News on May 16, 2022. View the full article here.
Song lab postdocs Ya-Dong Li, PhD, and Yan-Jia Luo, PhD, are co-first authors on the article. Other authors are Ze-Ka Chen, Luis Quintanilla, and Libo Zhang, of the Song Lab at UNC-Chapel Hill; Yoan Cherasse and Michael Lazarus at University of Tsukuba, Japan; and Zhi-Li Huang from Fudan University, China.
News-Media. net highlighted the paper May 16, 2022: “Targeting specific neurons modulates memory retrieval and alters anxiety-like behaviors in mice.”
Abstract
Adult hippocampal neurogenesis plays a critical role in memory and emotion processing, and this process is dynamically regulated by neural circuit activity. However, it remains unknown whether manipulation of neural circuit activity can achieve sufficient neurogenic effects to modulate behavior. Here we report that chronic patterned optogenetic stimulation of supramammillary nucleus (SuM) neurons in the mouse hypothalamus robustly promotes neurogenesis at multiple stages, leading to increased production of neural stem cells and behaviorally relevant adult-born neurons (ABNs) with enhanced maturity. Functionally, selective manipulation of the activity of these SuM-promoted ABNs modulates memory retrieval and anxiety-like behaviors. Furthermore, we show that SuM neurons are highly responsive to environmental novelty (EN) and are required for EN-induced enhancement of neurogenesis. Moreover, SuM is required for ABN activity-dependent behavioral modulation under a novel environment. Our study identifies a key hypothalamic circuit that couples novelty signals to the production and maturation of ABNs, and highlights the activity-dependent contribution of circuit-modified ABNs in behavioral regulation. (~Abstract excerpt from Nature Neuroscience, 25: 630–645, 2022.)
