Congratulations to Pharmacology graduate student Raquel C. Martinez Chacin, who is first author on a paper published in Nature Structural and Molecular Biology Journal! The paper by the Brown Lab in collaboration with the Emanuele Lab, Jean Cook Lab, and other researchers at UNC and the Research Institute of Molecular Pathology (IMP) in Vienna was published May 11, 2020!

Abstract
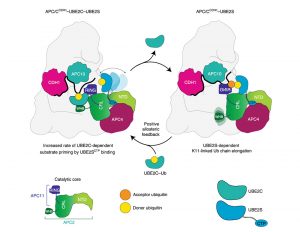
The interplay between E2 and E3 enzymes regulates the polyubiquitination of substrates in eukaryotes. Among the several RING-domain E3 ligases in humans, many utilize two distinct E2s for polyubiquitination. For example, the cell cycle regulatory E3, human anaphase-promoting complex/cyclosome (APC/C), relies on UBE2C to prime substrates with ubiquitin (Ub) and on UBE2S to extend polyubiquitin chains. However, the potential coordination between these steps in ubiquitin chain formation remains undefined. While numerous studies have unveiled how RING E3s stimulate individual E2s for Ub transfer, here we change perspective to describe a case where the chain-elongating E2 UBE2S feeds back and directly stimulates the E3 APC/C to promote substrate priming and subsequent multiubiquitination by UBE2C. Our work reveals an unexpected model for the mechanisms of RING E3–dependent ubiquitination and for the diverse and complex interrelationship between components of the ubiquitination cascade.
Significance: Dysregulation of polyubiquitination can lead to severe diseases such as cancer or developmental disabilities. Gaining a better understanding of how E2 and E3s collaborate and work together for polyubiquitination may lead to development of new therapeutics to treat such diseases.
Read the journal article on Nature Structural and Molecular Biology
Nicholas G. Brown and Michael J. Emanuele are co-corresponding authors on the paper. Other co-authors from UNC are: Jeanette G. Cook, Derek L. Bolhuis, Thomas Bonacci,Tatyana Bodrug, Gavin D. Grant, Katarzyna M. Kedziora, and Morgan E. Gibbs. Co-authors from other universities and research institutions are: Alban Ordureau and J. Wade Harper from Harvard University; and Florian Weissmann, Renping Qiao, amd Jan-Michael Peters of Research Institute of Molecular Pathology (IMP) in Vienna.
