Cyrus Vaziri, PhD
Professor
Areas of Interest
DNA damage; DNA replication; DNA repair; signal transduction; genome maintenance; carcinogenesis; cancer therapy
About
v
- Department Affiliations:
- Pathology and Lab Medicine; Biochemistry and Biophysics
My Research
Our overall goal is to elucidate mechanisms by which cells sense, repair and tolerate DNA damage, and to understand how loss of these ‘genome maintenance’ mechanisms leads to disease.
Human cells are frequently exposed to DNA-damaging agents from environmental and endogenous sources. Failure to tolerate DNA damage can lead to loss of cell viability and altered homeostasis (e.g. bone marrow failure syndromes arising from reduced DNA damage tolerance of hematopoietic progenitors). Moreover, failure to replicate and repair damaged DNA accurately can lead to genomic instability and cancer. Indeed many cancer-propensity syndromes result from defective DNA repair.
DNA repair is also very relevant to cancer treatment: radiotherapy and many chemotherapeutic drugs kill cancer cells by causing irreparable DNA damage. Unfortunately, cancer cells can acquire resistance to chemotherapy and radiotherapy via activation of DNA repair pathways. Therefore, DNA repair proteins represent potentially valuable therapeutic targets in the cancer clinic.
Much of our research is focused on a DNA damage tolerance mechanism termed ‘Trans-Lesion Synthesis’ (TLS): In response to many DNA-damaging exposures, specialized TLS DNA polymerases are recruited to sites of DNA damage where they replicate and repair damaged DNA, thereby conferring DNA damage tolerance. However TLS is an inherently error-prone process and must be used sparingly to avoid genome instability. Our laboratory is interested in the signal transduction mechanisms that regulate TLS, promote genome maintenance, and guard against cancer in normal cells. We also seek to identify TLS inhibitors that will improve the effectiveness of DNA-damaging therapeutic agents against chemo-resistant cancer cells.
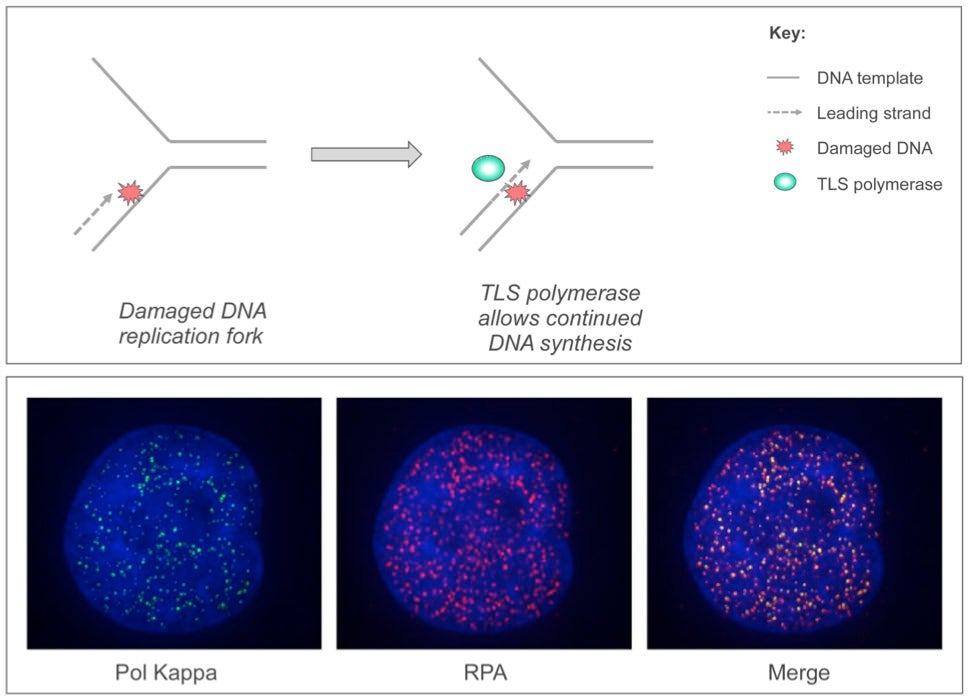
Figure Legend
Upper Panel: When cells acquire DNA damage (red ‘explosion’), specialized TLS DNA polymerases (green sphere) are recruited to DNA replication forks to allow completion of DNA synthesis, thereby conferring DNA damage tolerance. Lower Panel: Confocal microscopy images showing DNA damage-inducible nuclear co-localization of the TLS DNA polymerase Pol Kappa (green foci) with the DNA replication fork protein RPA (red foci).
