Janet Rubin MD
The Laboratory of Dr. Janet Rubin

| Faculty Homepage: | Divisional Home Page |
|---|---|
| Email: | jrubin@med.unc.edu |
| Appointments: | Professor, Division of Endocrinology/Metabolism Department of Medicine Professor in Pharmacology and Pediatrics Adjunct Professor, Bioengineering |
| Room: | 5032 Burnett Womack |
| Phone: | 919-966-6744 clinic patients use 919-484-1015 |
Skeletal Research
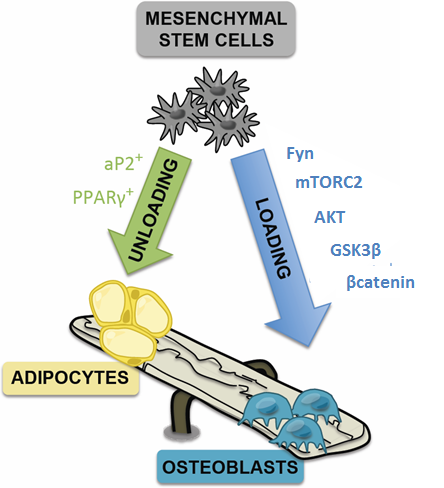
The skeleton is a complex tissue that is able to regulate its own mass and architecture to meet two critical and competing responsibilities: one structural and the other metabolic. The structure of bone is determined largely through its ability to respond to daily loading with intelligent remodeling. When mechanical signals are suppressed (no exercise, space travel, getting old) bone structure degenerates – and bone resorption outpaces bone formation. If skeletal degeneration is severe it will lead to catastrophic failure with fracture. Alternatively, mechanical signals arising from dynamic skeletal loading leads to bone formation, enhancing s the entry of mesenchymal stem cells into the osteogenic lineage. Our cellular and molecular biological investigations are aimed at understanding mechanical and hormonal control of bone remodeling.
We have concentrated on how loading directs the lineage of bone marrow mesenchymal stem cells, showing that multiple forces prevent adipogenesis, retaining the potential of the MSC to respond to signals that cause osteogenesis.
We have mapped the mechanical signal pathway that regulates MSC lineage decisions. In the case of substrate strain, the force is transmitted at focal adhesions, activating the pathway Fyn/FAK→mTORC2→ Akt→inhibit GSK3β→ βcatenin. Increased βcatenin is largely responsible for the anti-adipogenic signal.
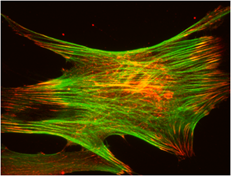
The same signaling pathway also stimulates cytoskeletal reorganization through activating RhoA activity – this generates new focal adhesion formation (and new signaling potential) and tension due to cytoskeletal connectivity, shown below in a mechanically strained cell.
Laboratory projects include defining:
- the synergy between cytoskeleton and signaling to control MSC lineage fate
- how low magnitude high frequency vibration controls MSC lineage
- how mTORC2 is activated by strain and vibration
- how MSC become osteocytes and respond to loading
- how exercise and diet control fat formation in bone marrow of running mice
Current members of our lab in 5030 Burnett-Womack:
- William Thompson PhD
- Gunes Uzer PhD
- Buer Sen MD
- Zhihui Xie MD
- Maya Styner MD (Endocrine faculty)
- Sherwin Yen MD
- Xin Wu
- Natasha Case PhD (also member of Dr Farshid Guilak’s laboratory at Duke)
- Kaitlyn Brobst, UNC undergraduate
- Gunis Uzer
Previous members have included Kornelia Galior (PhD candidate at Emory), Jacob Thomas (MD student), Emily Neely MD, James Meeker MD, Meiyun Ma MD, Minxou Zou MD, and Chris O’Conor (Guilak lab/Duke).
Click here to see some of us in the lab: Rubin Lab Folks
 We are grateful to NIAMS and NIBIB for our NIH support.
We are grateful to NIAMS and NIBIB for our NIH support.
Essential collaborations continue with:
- Dr. Clinton Rubin, Musculoskeletal Research, SUNY
- Dr. Ted Gross, Orthopedics, University of Washington
- Dr. Mark Horowitz, Orthopaedics, Yale University
- Dr. Keith Burridge, UNC Cell Biology
- Dr. Margaret Gourlay, UNC Family Medicine
Recent Publications
1. Ozcivici E, Luu YK, Adler B, Qin Y, Rubin J, Judex S, Rubin CT 2010 Mechanical signals as anabolic agents in bone, Nature Reviews in Rheumatology, 6:50-59.
2. Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J 2009 Mechanical loading regulates NFATc1 and b-catenin signaling through a GSK3b control node, J Biological Chemistry, 284:34607-34617
3. Case N, Zou M, Xie Z, Sen B, Styner M, O’Conor C, Horowitz M, Rubin J 2010 Mechanical activation of β-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. J Orthop Res. 28(11):1531-8.
4. Case N and Rubin J 2010 Prospects: β-catenin – a supporting role in the skeleton, J Cell Biochem. 110(3):545-53.
5. Styner M, Sen B, Xie Z, Case N, Rubin J 2010 Indomethacin promotes adipogenesis from MSC through PG independent mechanisms, J Cell Biochem. 111(4):1042-50
6. Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J 2011 Mechanical inhibition of adipogenesis achieved via a regenerated β-catenin signal is amplified by incorporating a refractory period, Journal Biomechanics 44(4):593-9
7. Gourlay ML, Preisser JS, Hammett-Stabler CA, Rubin J 2011 Follicle stimulating hormone is less important than weight and race in determining bone density in younger postmenopausal women, Osteoporosis Int 22:2699-708
8. Case N, Thomas J, Xie Z, Sen B, Styner M, Rowe D, Rubin J 2013 Mechanical input restrains PPARγ2 expression and action to preserve MSC multipotentiality Bone 52(1):454-64.
9. Sen B, Guilluy C, Xie Z, Case N, Styner M, Thomas J, Oguz I, Rubin C, Burridge K, Rubin J, 2011 Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells Stem Cell 29:1829–1836
10. Case N, Thomas J, Styner M, B Sen, Xie Z, Rubin J 2011 Mechanical activation of Akt via serine 473 phosphorylation requires mTORc2 in MSC. J Biol Chem 286(45):39450-6.
11. Duan J, Lee Y, Corey J, Gong J, Rojas M, Burk L, Willis M, Homeister J, Tilley S, Rubin J and Deb A 2013 Rib fractures and death due to deletion of osteoblast βcatenin in adult mice is rescued by corticosteroids, PLoS One, 8(2):355757
12. Styner M, Meyer MB, Galior K, Case N, Sen B, Xie Z, Thompson WR, Pike JW, Rubin J 2012 Mechanical strain downregulates C/EBPβ in MSC and decreases endoplasmic reticulum stress PLoS One 7(12):e51613
13. Thompson WR, Rubin CT, Rubin J 2012 Mechanical regulation of signaling pathways in bone, Gene 503(2):179-93
14. Sen B, Xie Z, Thomas W, Case N, Uzer G, Styner M, Rubin J 2013 mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow derived mesenchymal stem cells, JBMR in press
15. Thompson WR, Guilluy C, Case N, Styner M, Sen B, Xie Z, Burridge K, Rubin J 2013 Mechanically activated Fyn induces activation of RhoA through mTORC2 in mesenchymal stem cells, Stem Cells, in press

I am forever in debt to my colleagues at Emory/VAMC in Atlanta (2006!), including Dr. Xian Fan, Tamara Murphy and Jill Rahnert.