The Animal Models Core (AMC) offers a full range of CRISPR/Cas9 services for genome editing in mice, rats and cell lines. The core was an early adopter of the CRISPR system in 2013 and have now completed over 150 CRISPR projects.
Using the CRISPR System for Genome Editing in Mice and Rats:
The CRISPR system has revolutionized the production of genetically modified rodent models. CRISPR has several advantages over previous technologies for genome editing in mice and rats.
Pick Your Strain. First, CRISPR genome editing can be performed by delivering CRISPR reagents directly into embryos. This bypasses the need for embryonic stem cells and allows genome editing in the specific mouse or rat strain of interest. By creating the mutation directly in the strain of interest, there is no need for extensive backcrossing, saving months and years on project timelines. Founder animals are typically produced within 3-4 months, and first-generation heterozygous animals can be obtained within 6 months. New mutant models have been generated in multiple different mouse strains including C57BL/6J, FVB/NJ, BALB/cJ, SKH1 Hairless, 129S1/SvImJ, NRG Immunodeficient, and collaborative cross strains. In rats, CRISPR in Sprague Dawley, ZDF and Fischer 344 have been used.
Pick Your Mutation. Second, CRISPR-mediated genome editing does not require a selectable marker, allowing mutations to be inserted without additional extraneous sequence changes. This allows more precise human disease modeling such as insertion of single nucleotide polymorphisms, point mutations or deletions.
Pick 2 Mutations. Third, the CRISPR system is very efficient. The high efficiency enables introduction of more than one mutation at a time. This is especially useful for simultaneously knocking out two genes on the same chromosome or making 2 different modifications in the same gene. It also allows the creation of large deletions in the genome, such as the deletion of a whole gene cluster.
In summary, CRISPR allows rapid creation of better animal models for clients’ research programs.
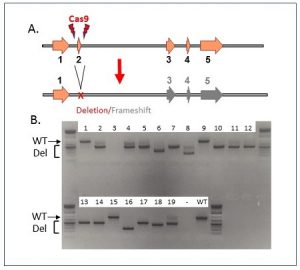 CRISPR-Mediated Gene Deletion in Mice. A. Schematic of strategy for gene inactivation by CRISPR-mediated exon deletion. Cas9 is targeted to cut on each side of a critical exon. Exon deletion causes protein frameshift, resulting in loss of gene function. B. Genotyping data from CRISPR deletion founders. Embryos were microinjected with Cas9 protein, 2 guide RNAs and a donor oligonucleotide designed to facilitate precise joining of the 2 Cas9 cut sites. Resulting animals were genotyped by PCR amplification with primers spanning the deletion site. Positions of wild-type and deletion bands are indicated. 16 of 19 animals show evidence of CRISPR-mediated deletions.
CRISPR-Mediated Gene Deletion in Mice. A. Schematic of strategy for gene inactivation by CRISPR-mediated exon deletion. Cas9 is targeted to cut on each side of a critical exon. Exon deletion causes protein frameshift, resulting in loss of gene function. B. Genotyping data from CRISPR deletion founders. Embryos were microinjected with Cas9 protein, 2 guide RNAs and a donor oligonucleotide designed to facilitate precise joining of the 2 Cas9 cut sites. Resulting animals were genotyped by PCR amplification with primers spanning the deletion site. Positions of wild-type and deletion bands are indicated. 16 of 19 animals show evidence of CRISPR-mediated deletions.
CRISPR Genome Editing in Cell Lines:
In addition to generating animal models, AMC now also offers CRISPR genome editing services in cell lines. They have used CRISPR for genome editing in several cell lines including mouse embryonic stem (ES) cells, human induced pluripotent cells (iPSCs), HEK293, OCI-AML-3 and primary mouse and rat embryonic fibroblast cells.
New Equipment and Technology:
The Animal Models Core recently received funding from the Core Facilities Advocacy Committee (CFAC) to obtain a NEPA21 Electro-Kinetic Transfection System for embryo electroporation. CRISPR genome editing has historically been performed by microinjection of CRISPR reagents in mouse and rat embryos. Embryo microinjection is highly technical and time consuming, requiring tremendous skill to minimize damage to the embryos while delivering the CRISPR reagents. Additionally, embryos from some mouse and rat strains are extremely sensitive to microinjection-induced damage, making genome editing difficult in those strains. The NEPA21 unit allows introduction of CRISPR reagents by electroporation. Multiple embryos can be electroporated simultaneously, dramatically reducing time requirements for reagent delivery. Electroporation is also less damaging than microinjection, allowing better embryo survival rates and facilitating genome editing in sensitive strains. Electroporation technology is currently used for production of knockouts, multiple gene deletions and small insertion mutations. The availability of this technology has increased our throughput and flexibility.
Visit the Animal Models Core website for information on their full range of services