Research
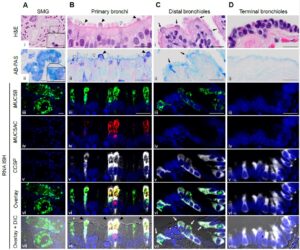
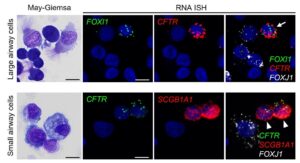
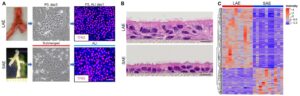
We inhale about 10,000 L of air to take oxygen into our bodies every day. Along with the inhaled air, numerous pathogens, chemical pollutants, and other irritants are inhaled, which could pose potential life-threatening risks to our lungs. However, our lungs are protected by mucociliary clearance (MCC), a critical innate defense mechanism that is important for maintaining lung health. The Okuda lab’s overall research interest focuses on how the MCC system is regulated to maintain homeostasis in the lung and how it fails in muco-obstructive lung diseases, including cystic fibrosis (CF), asthma, and COPD. Our previous work successfully characterized the regional expression patterns of major airway secretory mucins, MUC5AC/MUC5B, and CFTR/ionocytes in normal and CF human airways (Am J Respir Crit Care Med, 2019, 2021). These investigations provide insight into the small airway region (< 2 mm in diameter) as a critical site for pathogenesis of muco-obstructive lung diseases. We have developed a microdissection technique for human small airways and established in vitro and explant small airway epithelial cell cultures. We have combined these culture systems with single-cell-based omics approaches and gene editing technologies to understand cellular biology and physiology of the human small airways. In response to the emergent situation caused by the SARS-CoV-2 pandemic, the Okuda lab has also been actively involved in COVID-19 research.