Publications
Complete List of Published Work in Hantman Bibliography
Selected Papers
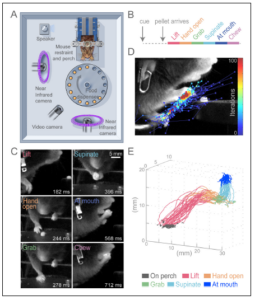 Guo, J.-Z., Graves, A. R., Guo, W. W., Zheng, J., Lee, A., Rodríguez-González, J., Li, N., Macklin, J. J., Phillips, J. W., Mensh, B. D., Branson, K. & Hantman, A. W. Cortex commands the performance of skilled movement. Elife 4, e10774 (2015). Guo, J.-Z., Graves, A. R., Guo, W. W., Zheng, J., Lee, A., Rodríguez-González, J., Li, N., Macklin, J. J., Phillips, J. W., Mensh, B. D., Branson, K. & Hantman, A. W. Cortex commands the performance of skilled movement. Elife 4, e10774 (2015).
Motor cortex is thought to be crucial for voluntary skilled movements like reaching. Yet when and to what degree motor cortex is necessary for such behaviors remain poorly understood. Much of what we know about the role of cortex came from surgical lesions, which have low temporal resolution and also permit compensation leading to underestimation of behavioral effects. The rapid and reversible nature of optogenetics offers a method to test the moment-by-moment role of cortex during behavior while minimizing compensation. To take full advantage of the optogenetic toolbox, we developed a new skilled movement paradigm in which head-fixed mice responded to a cue and reached out to grab a remembered target in the dark. Head-fixation offered many advantages including well-timed, stereotyped, accurate, and precise behavior; movements largely restricted to the arm; ability to use computer vision techniques to semi-automatically track fine aspects of the behavior; and the ability to easily manipulate different regions of the nervous system. Using these methods, we discovered that optogenetic suppression of motor cortex made it nearly impossible for the mouse to initiate a reach and could also halt ongoing movement. While this demonstration of the necessity of cortex for learned motor programs answers a long-standing open question, something else fascinating occurred when inhibition terminated. At that time, all trained animals in this study reached and grabbed for the pellet location, even if no cue or pellet was presented. Therefore, termination of inhibition itself is sufficient to specifically trigger the learned multi-step behavior. Command systems are strictly defined as neural components necessary and sufficient to evoke a behavior. By satisfying these criteria, we demonstrate that cortex commands the continuous performance of skilled movement. This work emphasizes the utility of the mouse model system for exploring the causal role of motor cortex for skilled behaviors. |

To control movement, motor cortex must generate activity patterns over time. These patterns result from interaction between local cortical circuitry and inputs from other brain regions, like thalamus. One possibility is that for well-trained stereotyped movements, cortex acts autonomously; once properly initialized by its inputs, cortex can produce the activity patterns on its own. In a contrary view, cortex can only produce the appropriate pattern during movement with time-varying inputs. Distinguishing these possibilities would indicate whether the skilled motor program is stored in motor cortex or distributed across multiple brain areas. The autonomous view of motor cortex has gained favor in the primate reaching field, but a causal test dissociating the role of the intrinsic properties of cortex from its inputs is lacking. To investigate the nature of the pattern generator underlying our cortically dependent reach-to-grab task, we first recorded rich neural activity patterns in layer 5 of motor cortex that correlated with various aspects of the behavior. To directly test the role of the inputs, we perturbed motor thalamus, one of the largest sources of external drive to cortex. Silencing motor thalamus greatly perturbed cortical activity patterns and blocked the initiation and ongoing progression of the behavior. Similarly, activation of motor thalamus could steer cortical dynamics off their normal course, leading to aberrant arm movements. Strong relationships between simultaneously recorded thalamic and cortical activity provided evidence that much of the temporal pattern in cortex was already present in its inputs. Therefore, unlike spinal pattern generators for locomotion, motor cortex is not an autonomous pattern-generating machine but must cooperate with broadly distributed brain networks to orchestrate movement. These findings influenced us to refocus our attention away from the level of cell-types or even brain regions and towards global brain dynamics. |
|
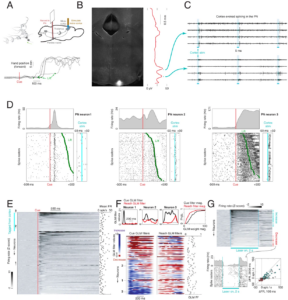 Guo, Jian-Zhong, Britton A. Sauerbrei, Jeremy D. Cohen, Matteo Mischiati, Austin R. Graves, Ferruccio Pisanello, Kristin M. Branson, and Adam W. Hantman. 2021. “Disrupting Cortico-Cerebellar Communication Impairs Dexterity.” eLife 10 (July). doi:10.7554/eLife.65906. Guo, Jian-Zhong, Britton A. Sauerbrei, Jeremy D. Cohen, Matteo Mischiati, Austin R. Graves, Ferruccio Pisanello, Kristin M. Branson, and Adam W. Hantman. 2021. “Disrupting Cortico-Cerebellar Communication Impairs Dexterity.” eLife 10 (July). doi:10.7554/eLife.65906.
Cerebral cortex and cerebellum, two of the largest structures in mammalian brains, are each thought to be crucial for planning and execution of skilled movements. While these regions each participate in many circuits, they also bidirectionally interact with one another. To explore cortico-cerebellar interactions, we investigated the pontine nuclei, which connect cortex to cerebellum. Pontine function has been difficult to study partly because its anatomical position at the bottom of the brain hinders recording and partly because classical lesions are nonselective due to unavoidable collateral damage to passing fiber tracts. To overcome these obstacles, we tested pontine nuclei function during prehension in mice, which allows us to take advantage of the cell-type and temporal resolution of optogenetics. High-density recordings from opto-tagged cortical-recipient pontine neurons revealed unexpected multimodal activity patterns (e.g. correlated to both cue and reach). Optogenetic perturbations of the pontine nuclei, using transgenic lines we developed, produced deficits in skilled control (accuracy, precision, and/or timing) while preserving the basic capacity to execute the movement. This contribution to skill is the first major motor function definitely ascribed to the pontine nuclei, and the selectivity of the behavioral effect surprised us. To further dissect the circuit, during pontine perturbations, we made electrophysiological measurements throughout the cortico-cerebellar loop both at rest and during behavior. Cerebellar cortex activity was dominated by the pontine perturbation in both conditions. In contrast, in the deep cerebellar nuclei and in cerebral cortex, the normal activity pattern underlying prehension was largely recapitulated during behavior. Nevertheless, persistent neural effects in both regions could decode the observed behavioral deviations. We concluded that motor control in the face of aberrant pontocerebellar inputs is likely robustly maintained by filtering of cerebellar cortical output and the convergence from other input systems. Thus, the cortico-cerebellar system fine-tunes the neural dynamics underlying prehension. |
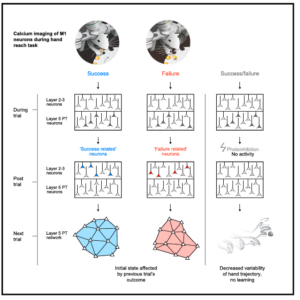 Levy, S., Lavzin, M., Benisty, H., Ghanayim, A., Dubin, U., Achvat, S., Brosh, Z., Aeed, F., Mensh, B. D., Schiller, Y., Meir, R., Barak, O., Talmon, R., Hantman, A. W.* & Schiller, J.* Cell-Type-Specific Outcome Representation in the Primary Motor Cortex. Neuron (2020). doi:10.1016/j.neuron.2020.06.006 Levy, S., Lavzin, M., Benisty, H., Ghanayim, A., Dubin, U., Achvat, S., Brosh, Z., Aeed, F., Mensh, B. D., Schiller, Y., Meir, R., Barak, O., Talmon, R., Hantman, A. W.* & Schiller, J.* Cell-Type-Specific Outcome Representation in the Primary Motor Cortex. Neuron (2020). doi:10.1016/j.neuron.2020.06.006
Adaptive movements are critical for all aspects of survival and happen constantly, whether we learn new behaviors, respond to changes in our environment, or refine existing skills. To adapt, the brain must have access to signals that indicate if an action is performed correctly or not. Such signals should inform the pattern generators to either reinforce previous motor commands or modify them to produce better future performance. While outcome signals are strongly implicated in reinforcement learning theories, the underlying neuronal circuits that support such signals remain practically unexplored, particularly in areas directly involved in motor control, such as primary motor cortex. To test if outcome signals are present in motor cortex, we used our reach-grab-delivery task in head-fixed mice combined with cell-type-specific, chronic, two-photon, calcium imaging and behavioral and optogenetic manipulations. We identified new functional cell types, “success” and “failure” reporting neurons which are found selectively in layer 2/3 of motor cortex. These neurons do not report reward or expectation of reward, as seen in other prefrontal cortical regions. Instead, they report the end result of the motor performance, and thus can serve as task-specific reinforcement signals. Before the next trial, outcome signals are erased in layer 2/3 but emerge and are maintained in the network initial state of layer 5 pyramidal tract neurons. Using post-movement optogenetic perturbation of motor cortex, we show that memory of outcome in the intertrial activity is critical for learning changes in the task. Access to such teaching signals might explain why neural dynamics underlying skilled behaviors necessarily route through motor cortex. Although many questions remain about the origin and downstream consequences of these signals, we now have an entry point to understand how outcome signals are used for adapting skilled motor programs. |
|
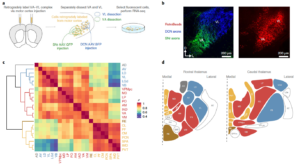
The thalamus is a complex switchboard composed of many nuclei, which controls the flow of all sensory information to multiple forebrain regions. Attempts to discern the logic of thalamic pathway organization have focused on a fraction of these thalamic nuclei and used sparse morphological, physiological, or molecular data. Molecular efforts typically led to a dichotomous view (e.g. core vs matrix) of the cellular diversity of thalamus. Here, we provide an unbiased characterization of nearly the entire thalamus (30/33 of the anatomically defined thalamic nuclei) by using dense single-cell and pooled-cell transcriptomic information. Through this effort we visualized the full ‘molecular space’ of transcriptional identities in thalamus and defined a low-dimensional structure that captured much of the transcriptional variance. Instead of two major molecular cell types, we found that thalamus is composed of three major profiles bridged by intermediate cell types to form a spectrum. Anatomically, the cell-types were typically arranged along the medial-lateral axis of thalamus. Genes differentially expressed across the three thalamocortical cell types raise hypotheses about their functional differences. For example, we found receptors and ion channels to be major classes of differentially expressed genes and showed corresponding systematic variation of intrinsic and synaptic properties of the three major profiles in motor thalamus. ‘Marker’ genes with binary expression properties are useful for generating tools that will facilitate experimental investigation of these thalamocortical cell types. Each neocortical region (e.g., motor cortex) samples inputs across its own dedicated spectrum of thalamic types. Therefore, we are now in position to dissect the role of each thalamic channel in motor cortical dynamics and their relationship to behavior. Extending this strategy, we believe this new classification of thalamocortical systems will also be useful for exploring perceptual and cognitive functions of cortex. |