Skeletal Muscle
Regulation of Skeletal Muscle Development and Myoblast Fusion
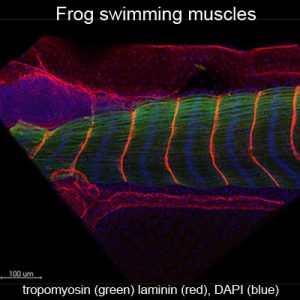
Unlike heart, mammalian skeletal muscle is highly plastic and has the capacity to regenerate after injury. In spite of this fact, muscle wasting does occur and is particularly debilitating in the elderly, in cancer patients, and in patients with congenital muscular dystrophies. While enhancing muscle mass will undoubtedly have a positive impact on morbitity and mortality associated with these diseases, current therapeutic approaches need improvement. We identified a protein termed GRAF (a GTPase activating protein for Rho) a FAK binding partner and component of adhesive complexes that is particularly abundant in developing mammalian skeletal muscle undergoing fusion to form multinucleated muscle fibers. Depletion of GRAF in developing Xenopus laevis tadpoles induced a highly-penetrable dystrophic phenotype that included a pronounced swimming defect accompanied by marked degeneration of muscle fibers, while ectopic expression of Graf in cultured mouse myoblasts induced remarkable muscle cell fusion. Collectively, these studies highlight the exciting possibility that targeting GRAF expression to failing muscle fibers may enhance muscle development and restore muscle function in a multitude of muscle wasting disorders. We now seek to identify the mechanisms underlying GRAF-dependent myotube formation, to test the efficacy of GRAF to counter muscle wasting, and to evaluate whether mis-regulation of GRAF contributes to the pathogenesis of congenital muscular dystrophies.