Specialty Areas:
Regulation of mucin gene expression in health and muco-obstructive pulmonary diseases.
Chronology:
BS, Biotechnology, 1999, College of Bioscience and Biotechnology, Yangzhou University, Jiangsu, China.
MS, Zoology, 2002, College of Veterinary Medicine, Yangzhou University, Jiangsu, China.
PhD, Molecular and Developmental Biology Graduate Program, 2010, Cincinnati Children’s Hospital Medical Center, University of Cincinnati.
Postdoctoral Fellow, 2010-2012, Division of Neonatology and Pulmonary Biology, Cincinnati Children’s Hospital Medical Center.
Postdoctoral Fellow, 2013-2014, Cystic Fibrosis and Pulmonary Research Center, University of North Carolina at Chapel Hill.
Research Associate, 2014-2020, Marsico Lung Institute/Cystic Fibrosis and Pulmonary Research Center, University of North Carolina at Chapel Hill.
Assistant Professor, 2020-now, Marsico Lung Institute/Cystic Fibrosis and Pulmonary Research Center, University of North Carolina at Chapel Hill.
Research Focus:
Mucus hyperconcentration is the hallmark of chronic airway inflammatory diseases including asthma, chronic bronchitis, COPD and CF. Excessive secretion of mucin by respiratory epithelia without sufficient hydration causes mucus dehydration, depletion of airway surface liquid volume and impaired mucociliary clearance, which in turn result in mucus obstruction, airflow restriction, chronic infection and inflammation and loss of lung structure and function.
Epithelial cells that line the conducting airways provide a physical barrier and initiate innate immune responses to the inhaled particles, microbes, and allergens by actively participating mucociliary clearance to remove them from the airways. Furthermore, epithelial cells synthesize chemokines, cytokines, and growth factors to promote migration and activation of professional immune cells to sites of injury following exposure to aerosol insults. Regulation of mucus production and innate immune responses is controlled by a complex transcriptional network that is comprised of SPDEF, FOXA3, FOXA2, NKX2-1 and the recently discovered spliced-XBP1. This process is also mediated by activation of epithelial cell surface receptors including IL4Rα, IL1R1, EGFR, TLRs, and ER membrane resident ER stress sensor protein ERN2 (IRE1β) following inflammation and injuries.
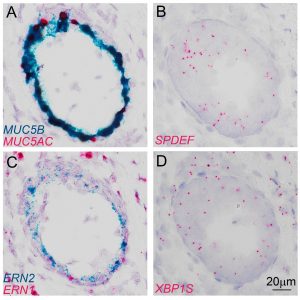
Our research interests are focused on understanding the critical roles of SPDEF, ERN2 and spliced-XBP1 in mediating mucus production/secretion and inflammatory responses during Th2 (by IL-13, IL-4 cytokines in asthma) and non-Th2 dominated inflammation (by IL-1β and IL-1α cytokines in CF). Further, we are also interested in understanding regulation of the MUC5B gene expression, which can be influenced by its promoter polymorphism rs35705950 locus that is associated with pathogenesis of IPF. We use both transgenic/knockout mouse models, primary normal and diseased human bronchial epithelial cells coupled with CRISPR-Cas9 technology to address the key roles of transcription factors in respiratory epithelial cells that regulate secretions of mucus and ion/water, and innate host defense that are highly relevant to pathogenesis of chronic muco-obstructive lung diseases.
Selected Bibliography:
- Hsu AP, Korzeniowska A, Aguilar CC, Gu J, Karlins E, Oler AJ, Chen G, Reynoso GV, Davis J, Chaput A, Peng T, Sun L, Lack JB, Bays DJ, Stewart ER, Waldman SE, Powell DA, Donovan FM, Desai JV, Pouladi N, Long Priel DA, Yamanaka D, Rosenzweig SD, Niemela JE, Stoddard J, Freeman AF, Zerbe CS, Kuhns DB, Lussier YA, Olivier KN, Boucher RC, Hickman HD, Frelinger J, Fierer J, Shubitz LF, Leto TL, Thompson III GR, Galgiani JN, Lionakis MS, Holland SM. Immunogenetics associated with severe coccidioidomycosis. JCI Insight. 2022 Sep 27:e159491. doi: 10.1172/jci.insight.159491. Epub ahead of print. PMID: 36166305.
- Kato T, Asakura T, Edwards CE, Dang H, Mikami Y, Okuda K, Chen G, Sun L, Gilmore RC, Hawkins P, De la Cruz G, Cooley MR, Bailey AB, Hewitt SM, Chertow DS, Borczuk AC, Salvatore S, Martinez FJ, Thorne LB, Askin FB, Ehre C, Randell SH, O’Neal WK, Baric RS, Boucher RC; NIH COVID-19 Autopsy Consortium. Prevalence and Mechanisms of Mucus Accumulation in COVID-19 Lung Disease. Am J Respir Crit Care Med. 2022 Jul 11. doi: 10.1164/rccm.202111-2606OC. Epub ahead of print. PMID: 35816430.
- Okuda K, Dang H, Kobayashi Y, Carraro G, Nakano S, Chen G, Kato T, Asakura T, Gilmore RC, Morton LC, Lee RE, Mascenik T, Yin WN, Barbosa Cardenas SM, O’Neal YK, Minnick CE, Chua M, Quinney NL, Gentzsch M, Anderson CW, Ghio A, Matsui H, Nagase T, Ostrowski LE, Grubb BR, Olsen JC, Randell SH, Stripp BR, Tata PR, O’Neal WK, Boucher RC. Secretory Cells Dominate Airway CFTR Expression and Function in Human Airway Superficial Epithelia. Am J Respir Crit Care Med. 2021 May 15;203(10):1275-1289. PMID: 33321047. PMCID: PMC8456462.
- Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon III KH, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Fulcher L, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O’Neal WK, Randell SH, Boucher RC, Baric RS. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020 Jul 23;182(2):429-446.e14. PMID: 32526206; PMCID: PMC7250779.
- Chen G, Sun L, Kato T, Okuda K, Martino MB, Abzhanova A, Lin JM, Gilmore RC, Batson B, Volmer AS, O’Neal YK, Dang H, Deng Y, Randell SH, Button B, Livraghi-Butrico A, Kesimer M, Ribeiro CMP, O’Neal WK, Boucher RC. IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J Clin Invest. 2019 Oct 1;129(10):4433-4450. PMID: 31524632; PMCID: PMC6763234.
- Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, Martino MB, Dang H, Abzhanova A, Lin JM, Hull-Ryde EA, Volmer AS, Randell SH, Livraghi-Butrico A, Deng Y, Scherer PE, Stripp BR, O’Neal WK, Boucher RC. XBP1S Regulates MUC5B in a Promoter Variant-Dependent Pathway in IPF Airway Epithelia. Am J Respir Crit Care Med 2019 Jul 15;200(2):220-234. PMID: 30973754; PMCID: PMC6635783.
- Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, Radicioni G, Kesimer M, Chua M, Dang H, Livraghi-Butrico A, Ehre C, Doerschuk CM, Randell SH, Matsui H, Nagase T, O’Neal WK, Boucher RC. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am J Respir Crit Care Med 2019 Mar 15;199(6):715-727. PMID: 30352166; PMCID: PMC6423099.
- Chen G, Volmer AS, Wilkinson KJ, Deng Y, Jones LC, Yu D, Bustamante-Marin XM, Burns KA, Grubb BR, O’Neal WK, Livraghi-Butrico A, Boucher RC. Role of Spdef in the Regulation of Muc5b Expression in the Airways of Naïve and Muco-obstructed Mice. Am J Respir Cell Mol Biol 2018; 59(3): 383-396. PMID: 29579396; PMCID: PMC6189647.
- Yu D, Saini Y, Chen G, Ghio AJ, Dang H, Burns KA, Wang Y, Davis RM, Randell SH, Esther CR, Jr., Paulsen F, Boucher RC. Loss of beta Epithelial Sodium Channel Function in Meibomian Glands Produces Pseudohypoaldosteronism 1-Like Ocular Disease in Mice. Am J Pathol 2018; 188: 95-110. PMID: 29107074; PMCID: PMC5745530.
- Donoghue LJ, Livraghi-Butrico A, McFadden KM, Thomas JM, Chen G, Grubb BR, O’Neal WK, Boucher RC, Kelada SNP. Identification of trans Protein QTL for Secreted Airway Mucins in Mice and a Causal Role for Bpifb1. Genetics 2017; 207: 801-812. PMID: 28851744; PMCID: PMC5629340.
- Esther CR, Jr., Hill DB, Button B, Shi S, Jania C, Duncan EA, Doerschuk CM, Chen G, Ranganathan S, Stick SM, Boucher RC. Sialic acid-to-urea ratio as a measure of airway surface hydration. American journal of physiology Lung cellular and molecular physiology 2017; 312: L398-L404. PMID: 28062483; PMCID: PMC5374301.
- Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest 2015; 125: 2021-2031. PMID: 25866971; PMCID: PMC4463206.
- Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 2014; 189: 301-313. PMID: 24392884; PMCID: PMC3977731.
- Ren X, Shah TA, Ustiyan V, Zhang Y, Shinn J, Chen G, Whitsett JA, Kalin TV, Kalinichenko VV. FOXM1 promotes allergen-induced goblet cell metaplasia and pulmonary inflammation. Mol Cell Biol 2013; 33: 371-386. PMID: 23149934; PMCID: PMC3554115.
- Noah TK, Lo YH, Price A, Chen G, King E, Washington MK, Aronow BJ, Shroyer NF. SPDEF functions as a colorectal tumor suppressor by inhibiting beta-catenin activity. Gastroenterology 2013; 144: 1012-1023 e1016. PMID: 23376423; PMCID: PMC3738069.
- Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol 2013; 183: 35-48. PMID: 23665202; PMCID: PMC3702735.
- Wang IC, Snyder J, Zhang Y, Lander J, Nakafuku Y, Lin J, Chen G, Kalin TV, Whitsett JA, Kalinichenko VV. Foxm1 mediates cross talk between Kras/mitogen-activated protein kinase and canonical Wnt pathways during development of respiratory epithelium. Mol Cell Biol 2012; 32: 3838-3850. PMID: 22826436; PMCID: PMC3457538.
- Korfhagen TR, Kitzmiller J, Chen G, Sridharan A, Haitchi HM, Hegde RS, Divanovic S, Karp CL, Whitsett JA. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc Natl Acad Sci U S A 2012; 109: 16630-16635. PMID: 23012424; PMCID: PMC3478616.
- Maeda Y, Chen G, Xu Y, Haitchi HM, Du L, Keiser AR, Howarth PH, Davies DE, Holgate ST, Whitsett JA. Airway epithelial transcription factor NK2 homeobox 1 inhibits mucous cell metaplasia and Th2 inflammation. Am J Respir Crit Care Med 2011; 184: 421-429. PMID: 21562130; PMCID: PMC3175541.
- Chen G, Wan H, Luo F, Zhang L, Xu Y, Lewkowich I, Wills-Karp M, Whitsett JA. Foxa2 programs Th2 cell-mediated innate immunity in the developing lung. J Immunol 2010; 184: 6133-6141. PMID: 20483781.
- Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 2009; 119: 2914-2924. PMID: 19759516; PMCID: PMC2752084.
- Maeda Y, Suzuki T, Pan X, Chen G, Pan S, Bartman T, Whitsett JA. CUL2 is required for the activity of hypoxia-inducible factor and vasculogenesis. J Biol Chem 2008; 283: 16084-16092. PMID: 18372249; PMCID: PMC2414293.
- Park K-S, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana Hershey GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 2007; 117: 978-988. PMID: 17347682; PMCID: PMC1810569.
A full list of Gang Chen’s publication can be found at the following link:
https://www.ncbi.nlm.nih.gov/myncbi/1NO-gAEzLz0kH/bibliography/public/

