Research
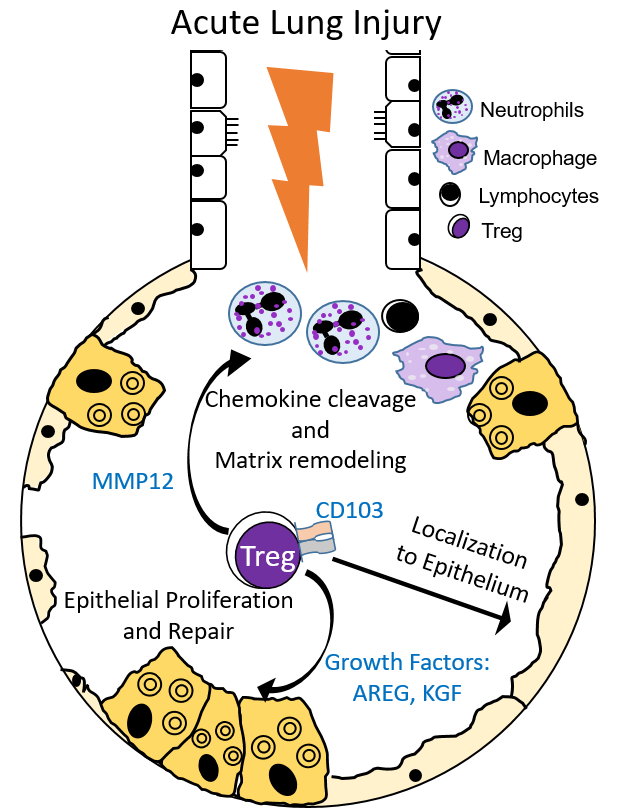
Our research interests focus on investigating the reparative processes critical to the resolution of acute lung injury. Acute events such as pneumonia, inhalational injury, trauma, or sepsis often damage the lung, impeding its primary function, gas exchange. The clinical syndrome these events can lead to is termed Acute Respiratory Distress Syndrome (ARDS). ARDS is a common pulmonary disease often seen and treated in intensive care units. Despite decades of research into the pathogenesis underlying the development of ARDS, mortality remains high. Our laboratory has built upon exciting observations by our group and others on the importance of how the lung repairs after injury. One type of white blood cell, the Foxp3+ regulatory T cell (Treg), appears essential in resolving ARDS in experimental models of lung injury–through modulating immune responses and enhancing alveolar epithelial proliferation and tissue repair. Importantly, Tregs are present in patients with ARDS, and our lab has found that subsets of Tregs may play a role in recovery from ARDS.
Current areas of investigation include:
- Examining the newly defined tissue reparative role of Tregs in lung resolution. Tregs express growth factors (AREG, KGF), which play a role in promoting lung injury resolution of the alveolar epithelium.
- Investigating the impact of Treg-expressed CD103, which our work has demonstrated, helps retain this lymphocyte population to the lung epithelium during ALI.
- Determining the role of Treg-expressed matrix metalloproteinase 12 (MMP12) in regulating the turnover of the extracellular matrix, in processing chemotactic signals that promote resolution of acute lung injury, and in other described MMP functions. The localization of Tregs within the alveoli may result in specific and vital functions for Treg-expressed MMP12 within these microenvironments.
- Investigating the role of specific Treg populations during ALI resolution and testing the hypothesis that potential mechanisms include the proliferation of lung-resident Tregs (either thymically-derived or peripherally-induced) and recruitment of Tregs from sites outside the lung.
- Determining if resolution can be accelerated by enhancing Treg expansion through proliferation, retention, stability, and/or survival. We are testing the hypothesis that a more robust Treg response accelerates the resolution process and improves the quality of lung repair.