Carrie Wilczewski, of Dr. Frank Conlon’s lab, is conducting studies that focus on visualizing and quantifying alterations in heart development in embryonic models of congenital heart disease. (Click Title to read more)
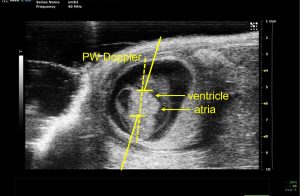
The Conlon group was seeking ways to assess the cardiac function during early developmental stages in the embryonic mouse models of congenital heart disease (CHD). While morphologic studies can be done on fixed embryo samples, assessing dynamic process of cardiac function in vivo is much more challenging. The size of the mouse heart at the gestation day (E)10.5 is only about 1 mm in width. It beats at a rate of more than 150 beats per minute, and pumps blood at an average velocity of about 140 mm per second. Capturing such moving object within live animal requires both high special resolution and high temporal resolution if imaging tool is to be used.
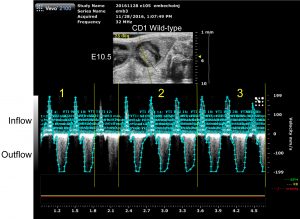
Working closely with the imaging core staff, Carrie has developed a series of imaging protocols using the ultrasound Vevo-2100 system to conduct non-invasive echocardiography on mouse embryo as young as E10.5 stage (Figure-1). The Vevo-2100 system is a dedicated animal ultrasound system with high resolution (down to 35 micron) capability. Using pulsed-waved (PW) Doppler imaging, she measured the velocity of blood flow at the atrioventricular (AV) canal to quantify differences in embryo cardiac function in mutant disease model and control mice (shown in Figure -2 and Video). Specifically, peak velocity at the AV canal and velocity time internal (VTI) were measured by PW Doppler to characterize each cardiac function.
Following Doppler imaging, ultrasound guided injection was performed to inject different colored fluorescent microspheres into the particular embryo so that individual embryos can be identified in postmortem analysis. Collectively, these methods allow the group to assess cardiac function at early embryo developmental stage non-invasively. In a model of non-cardiac myofibril gene misexpression, preliminary data have shown that mutant embryos show decreased cardiac function as measured by peak velocity and VTI compared to littermate controls.
With the novel developmental work, Carrie Wilczewski has won the VEVO Travel Award sponsored by VisualSonics to attend the Weinstein Cardiovascular Development and Regeneration Conference earlier this year in Columbus Ohio.