New Research in ANCA Vasculitis
This research is a successful collaboration between basic and translational scientists, epidemiologists, geneticists, and nephropathologists. The themes of the projects mirror questions that patients frequently ask: what causes their disease, what will make their disease better or worse, and what will assure that their disease goes into remission and even be cured? Dr. Ronald Falk is Principal Investigator.
Molecular Mechanisms of Disease
This project aims at unraveling the molecular underpinning of ANCA GN by elucidating genetic variation, epigenetic regulation of transcription, and clarification of the transcriptional profiles of the inflammatory cells engaged in the pathogenesis of ANCA vasculitis (neutrophils, monocytes, lymphocytes and low density neutrophils (LDNs). The Investigation team is led by Dominic Ciavatta, PhD and includes Ronald Falk, MD, and Meghan Free, PhD.
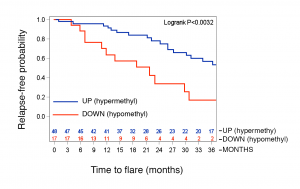
The role of epigenetic modifications in the progression of ANCA GN had not been previously established. We sought to understand how DNA methylation at various CpG sites within the autoantigen genes (MPO and PR3) might influence the disease severity or relapse rate of ANCA GN. These studies revealed that generally, MPO and PR3 are hypomethylated during active disease, but that methylation returned to normal levels during remission. Perhaps most interesting for future clinical studies was that certain patients who remained hypomethylated at the PRTN3 promoter had a significantly greater risk of relapse (hazard ratio 4.55) (J Am Soc Nephrol 2017; 28(4):1175-1187).
Recent studies have focused on identifying HLA variants of ANCA GN among our patient cohort and use the corresponding HLA to identify autoreactive T cells. Initial analysis of the North American GWAS sequenced HLA from over 450 patients revealed that the strongest association among ANCA GN was found for HLA-DPB1. HLA sequencing data of our ANCA-GN patient cohort identified the two most common HLA as DPB1*04:01 (65.6% carrier frequency) and DRB4*01:01 (59.4% carrier frequency), (Arthritis Rheumatol 2017; 69(5):1054-66). In collaboration with Bjoern Peters at La Jolla Institute for Allergy and Immunology and Eddie James and Bill Kwok at Benaroya Research Institute, PR3 and MPO peptides were identified that bind DPB1*04:01 and DRB4*01:01 and further studies were performed with patients carrying DPB1*04:01 or DRB4*01:01 to identify autoreactive T cells that recognize MPO peptides. The exciting result of these studies demonstrated that patients with ANCA GN have CD4+ T cell responses to a restricted region of MPO (J Autoimmun 2019; Epub ahead of print).
Immunodominant ANCA Autoantigens
This project seeks to identify immunodominant epitopes within ANCA autoantigens and to understand the modulation of adaptive immune responses on the risk and severity of ANCA GN. The investigation team is led by Meghan Free, PhD and includes Ronald Falk, MD and Donna Bunch, PhD.
Recent studies have built extensively on findings by Falk and colleagues of an immunodominant linear epitope of MPO. Utilizing an in-house ELISA directed against this linear epitope, it was learned that antibodies to this epitope are most detectable at onset of disease, and in some patients correlate with disease activity. The combination of B cells studies and HLA sequencing made it possible to create tetramers to study autoreactive T cells in the periphery of ANCA vasculitis patients (J Autoimmun 2019; Epub ahead of print). Collectively, these studies have provided the foundational knowledge to move forward towards antigen-specific therapies in select patients.
In collaboration with researchers at Vanderbilt University (Billy Hudson, PhD, and Scott McCall, MD, PhD), we have also investigated the antibody reactivity to peroxidasin which shares significant homology to MPO. These studies determined that certain patients with MPO-ANCA do have crossreactivity to peroxidasin. This knowledge will inform future studies into the inciting events that lead to initiation of ANCA GN and potentially anti-glomerular basement membrane (GBM) disease (J Am Soc Nephrol 2018; 29(11):2619-2625).
Adaptive and Innate Immune Responses/Animal Models of Disease
This project investigates the adaptive and innate immune responses that influence the risk, severity and clinicopathologic phenotypes of ANCA glomerulonephritis. The investigation team is led by J. Charles Jennette, MD and includes Donna Bunch, PhD and Dominic Ciavatta, PhD, Peiqi Hu, MD and Hong Xiao, MD.
A landmark in ANCA research has been the development of a mouse model that reproduces the human histopathology and definitively demonstrated that anti-MPO antibodies are indeed pathogenic. This mouse model allows us to study the role of various inflammatory pathways in the pathogenesis of ANCA vasculitis. Through a series of experiments in mice deficient in various components of the complement cascade, this model identified an important role of the alternative pathway of complement, but not the terminal membrane attack complex, in the pathogenesis of anti-MPO mediated disease. This work is the basis upon which novel therapeutic approaches for human ANCA vasculitis are being developed.
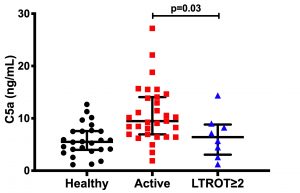
In collaboration with Eve Wu, MD (Allergy/Immunology and Pediatric Rheumatology), we have investigated the role of the complement cascade in human ANCA disease recently. These studies suggest that certain complement breakdown products (C3a, C5a, and sC5b-9) are elevated in active MPO-ANCA vasculitis. These same markers, plus C4d, were elevated in active PR3-ANCA vasculitis (Arthritis Rheumatol 2019; Epub ahead of print). Patients in long-term remission off therapy (LTROT) had significantly lower C5a than during active disease (see figure). Future studies will examine whether measures of complement activation are useful indicators of treatment response, relapse risk, and/or stable remission. Collectively, these joint human and animal studies underscore the importance of the complement cascade in ANCA vasculitis.
Kidney and Vascular Innovation Program
Vision
- A comprehensive, unique program and one of its kind that cares for the cardiovascular health of patients with CKD and ESKD
Mission
- To create an integrated bedside to bench to bedside program that targets cardiovascular health (cardiovascular disease and vascular access dysfunction) in CKD and ESKD patients through basic and translational science, technology development, clinical research and education/public policy inputs
- To develop novel and innovative biomarkers, drugs, devices, process of care and awareness/education pathways, that will improve cardiovascular health in CKD and ESKD patients
- To organize an integrated, patient centered clinical care pathway that is focused on cardiovascular health in CKD and ESKD patients and which could serve as test bed for the previously described innovations
Overview
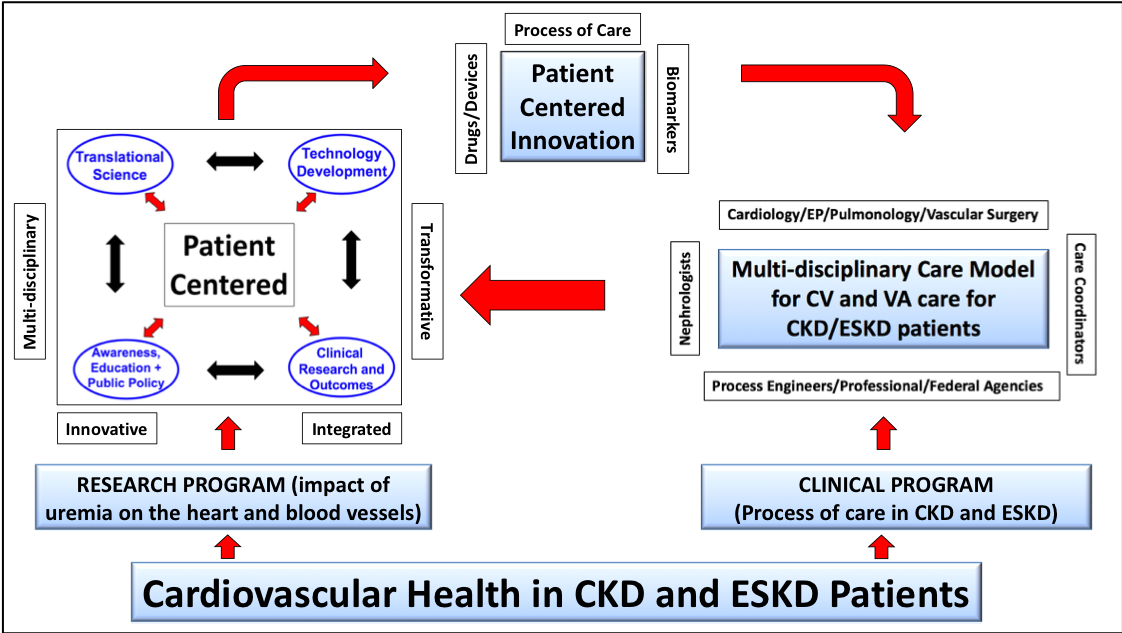
Cardiovascular disease and Vascular Access Dysfunction (both likely due to the negative impact of uremia on blood vessels and the heart) are responsible for the bulk of the morbidity, mortality, cost and quality of life issues for CKD and ESRD patients. Specifically, we spend 114 billion dollars per year on kidney care, of which 34 billion dollars is for the care of ESKD patients who have a dismal three year survival of 50%, mainly due to cardiovascular disease and vascular access complications. The Kidney and Vascular Innovation program will therefore be focused on overall cardiovascular health in these patients and will be comprised of two parallel but interlinked initiatives.
The translational initiative will focus on the impact of uremia on blood vessels and the heart. It will do so through an innovative, integrated, multi-disciplinary, transformative and patient centered approach, with four major and interconnected domains: (a) Translational Science (b) Technology Development (c) Clinical Research, Outcomes and Epidemiology and (d) Awareness, Education and Public Policy.
The clinical program will develop a process of care pathway for the vascular access and cardiovascular problems faced by CKD and ESKD patients. Key components will be the identification of subspecialists (cardiologists [heart failure, electrophysiology and interventional], pulmonologists, transplant and vascular surgeons, endocrinologists, podiatrists, radiologists and interventional nephrologists) who will collaborate with nephrologists and primary care physicians, to deliver high quality cardiovascular care to CKD and ESRD patients.
Most importantly, these two programs will interact in the following ways:
- The innovations from the translational program (biomarkers, drugs, devices, process of care and education/awareness pathways) will improve the cardiovascular health of patients in the clinical program
- The clinical program will serve as a test bed for the above innovations
- The clinical program will identify unmet clinical needs that will then be addressed by the translational program
- The co-localization of the two programs will result in an unprecedented opportunity for the teaching, training, professional growth and mentorship of a multidisciplinary cadre of healthcare professionals focused on cardiovascular health in CKD and ESKD patients
Contrast Enhanced Ultrasound work – Emily Chang, MD
Contrast enhanced ultrasound (CEUS), a specialized type of ultrasound that is safe for patients with kidney disease, is now available at UNC for the characterization of kidney and liver lesions as well as the evaluation of vesico-ureteral reflux in pediatrics. The efforts of Dr. Emily Chang and her team of investigators, including Dr. Kennita Johnson and Dr. Lauren Burke helped bring this technology to our patients.
Dr. Chang and her team are actively investigating different uses for CEUS, including imaging in patients with diabetes, kidney transplants and a rare genetic disease called von-Hippel Lindau that involves the kidney. This work is being conducted in both human and animal studies.
In addition, Dr. Chang has also recently expanded the scope of her work to investigate the use of point-of-care-ultrasound (POCUS) bedside ultrasound techniques in dialysis patients with the goal of understanding whether they have too much fluid, even after their dialysis treatment.
Progression of CKD – Thomas Hostetter
Dr. Thomas Hostetter’s research continues to focus on the progression of CKD, especially the role of bicarbonate therapy in mitigating progression, and identification of uremic toxins and markers of CKD progression and cardiovascular disease. He and his team concluded an NIH sponsored study of bicarbonate therapy in about 150 people with CKD, which was presented at the ASN Annual Meeting in 2018. He has over the last 2 years published on the effects of bicarbonate on general metabolism, cognitive function and production of fibrosing mediators. He works with industry in clinical trials of a bicarbonate enhancing polymer. He is a CoPI in the NIH supported Biocon II consortium, which seeks biomarkers of CKD progression and markers of CVD within CKD.
In June 2019, he concluded a 5 year term as chairman of an NIH study section for individual and institutional training grants. He serves as a Deputy Editor for the Journal of the American Society of Nephrology.
Insight to the heterogeneity of diabetic kidney disease (DKD) – Amy Mottl
Nephropathologic Mechanisms
Diabetic complications of the kidney are mediated in part by hyperglycemia and hypertension, but how these environmental triggers affect the pathobiology of the kidney varies between individuals. Amy Mottl, MD, MPH is working to publish results from a pilot study of 20 patients with type 2 diabetes and early-moderate DKD who underwent research protocol kidney biopsy. Some patients had changes in the glomeruli (filtration units) of the kidneys, while others had predominant changes in the vasculature (blood vessels) in the kidneys. Even amongst those with predominant vascular changes, some lacked the earliest manifestations of DKD, basement membrane thickening. This would indicate that their kidney damage might not have been due to diabetes but to hypertension, underscoring the need to consider the pathologic phenotype of DKD while investigating novel therapies.
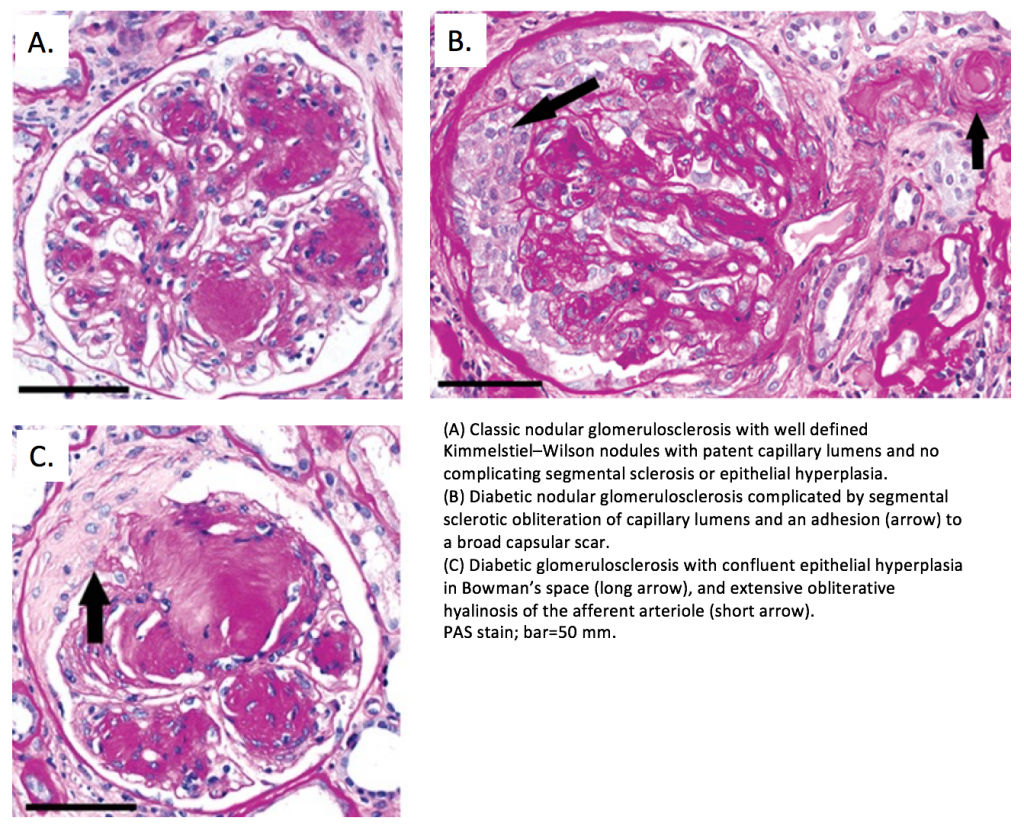
Severe DKD typically manifests with extensive expansion of the mesangial (collagenous) regions of the glomeruli, often forming nodules (Figure 1A). Dr. Mottl worked with J. Charles Jennette MD performed a retrospective study of specific nephropathologic changes in people with advanced DKD who had undergone clinical kidney biopsy to see which specific changes were most prognostic of progression to end-stage kidney disease. They published their findings in the Journal of the American Society of Nephrology (2018) demonstrating that segmental sclerosis (scarring in portions of the glomeruli – Figure 1B) and extracapillary hypercellularity (proliferation of cells within the urinary space – Figure 1C) are most predictive of kidney disease progression. Future work will focus on identifying biological mechanisms of these changes which may help to identify therapeutic targets for those patients at greatest risk.
DKD Treatments
Type 2 diabetes greatly increases the risk for both cardiovascular disease and chronic kidney disease. Therefore, it is especially important to protect the heart and kidney health of patients with type 2 diabetes. Dr. Mottl worked with investigators of the ACCORD Trial, a multifactorial intervention study of glycemic control (HbA1c <6% versus 7.0-7.9%) and blood pressure control (systolic blood pressure < 120 versus <140mmHg) in people with type 2 diabetes at high cardiovascular risk, to determine whether these intensive targets delayed progression of DKD. They found that intensive blood sugar control reduced the risk for macroalbuminuria (a high amount of protein excreted in the urine) over an average follow-up of 7.7 years, but it had no impact on more significant kidney outcomes such as serum creatinine doubling (a marker of worsening kidney function) or the need for dialysis or transplantation. Intensive control of blood pressure increased the risk for doubling of serum creatinine but did not increase the need for dialysis or transplantation. This work was presented at the 2018 American Society of Nephrology in a session “Best of 2018” in the ASN Journals, CJASN and JASN. Read article.
![Cumulative incidence of the primary composite kidney outcome (incident macroalbuminuria [UACR>300 mg/mg], doubling of creatinine, incident dialysis or all-cause mortality) according to randomization arm. (A) Glycemia trial (HbA1c: intensive arm <6.0% versus standard arm7.0%–7.9%) with the intensive arm showing reduced risk, (B) BP trial (systolic BP: intensive arm <120mmHg versus standard arm <140mmHg) with the intensive arm showing higher risk.](https://www.med.unc.edu/medicine/wp-content/uploads/sites/945/2019/10/blood-pressure-mottl-1024x335.png)
Understanding Pathobiology of Kidney Diseases – John Poulton, PhD
To understand the pathobiology of genetic and autoimmune diseases of the kidney, we must first understand how normal kidneys function, and then characterize how genetic or immune-related perturbations in patients lead to disease. Researchers and clinicians can then begin to rationally design and develop new treatments of kidney disease. There are many powerful tools available that can be harnessed and integrated to shed light on normal and pathological biological processes. John Poulton, PhD combines genetically tractable model organisms (e.g., zebrafish and Drosophila), high-throughput peptide screening platforms, and state-of-the-art microscopy to study the relationships between genes, cellular processes, and developmental biology—for example, see our recent work describing the transcriptional response to oxidative stress caused by loss of centrosomes. In collaboration with clinical researchers in the Division, Dr. Poulton is developing novel assays and animal models that will identify new genes involved in kidney development and function, as well as the genes and proteins that underlie the pathophysiology of various diseases of the kidney. Identification of these genes provides diagnostic potential as well as targets for therapeutic interventions.