Mucin Unpackaging/Maturation
Cystic Fibrosis in the lung is characterized by the failure of mucus clearance. Gel forming mucins MUC5B, in particular, is a major contributor to normal lung mucus, saliva and cervical mucus and is known to be an important product of the submucosal glands. Oligomeric gel forming mucins are packaged tightly into secretory granules by the direct involvement of calcium binding at low pH with the negative charge on their carbohydrate chain. The formation and organization of these exceptionally large molecules within the cell and the means and rapidity by which they unpack to form mucus gels is still unclear.
In the CF airways, the absence or malfunction of CFTR results in a volume depleted airway surface liquid (ASL) and a lower pH resulting in aberrant mucin maturation and perturbed rheology. Thus, it is important that we know how these factors effect mucin maturation into mucus after granular release, for the question bears directly on therapies for CF patients.
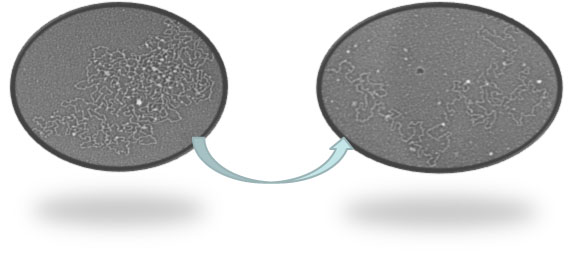
Our data on salivary MUC5B, employing transmission electron microscopy (EM), have shown that the expanding granular form of the mucin appears as small numbers of glycosylated mucin subunit chains connected around their N- and C-terminal protein regions. The appearance strongly suggests that the assembled mucins in a single granular form are built up from a number of small discrete spherical core structures (10-30 nm) containing 4 to 8 subunits. The organization of the mucins in this manner would be consistent with connecting a high number of large heavily glycosylated monomers while still permitting the rapid unfolding and hydration of these huge macromolecules. For the first time this provides some insight into how the huge carbohydrate domains are organized around the N-terminal globular protein domains and explains how these structures can expand so rapidly upon secretion from the cell.
However electron microscopy images of the same isolated MUC5B fraction from the CF saliva indicates the spherical proteins cores mentioned above are not so well organized/processed and up to 10 times bigger (100-300 nm) than the normal equivalent. Our hypothesis based upon recent studies is that in the absence of CFTR, mucin expansion/mucus maturation is impaired due to different post-secretory environment in CF mucus. This project focuses a wide- ranging assessment of mucin expansion and maturation in normal mucus and its alteration due to improper post secretory processing in CF mucus.
Support: Cystic Fibrosis Foundation KESIME14XX0 (Kesimer, PI)