Behavioral and Pharmacological Neurodynamics Lab
Left to right: Jon Harden, Rayya Jawad, Molly Crosswell, Dr. Donita Robinson, Dr. Alex Goméz-A, Dr. Carol Dannenhoffer, Meira Machado, Minna McFarland, Jhoan Aguilar, Dr. Victoria Macht (guest appearance).
The Robinson Lab studies brain mechanisms during motivated behavior with the aim to understand neuronal circuitry contributing to behavioral flexibility in typical and drug-exposed adolescents and adults. We use rats as a model for human development and drug exposure, which allows us to directly measure and manipulate brain activity. Our technical expertise is in vivo brain recordings coupled to behavior.
- With electrophysiology, we use a voltage follower to monitor action potentials of corticostriatal neurons, detected at chronically implanted microelectrode arrays (Figure 1).
- With electrochemistry, we use a current follower to monitor fluctuations in dopamine concentrations on a sub-second timescale, by using fast scan cyclic voltammetry at acutely implanted carbon-fiber microelectrodes (Figure 2).
- With optogenetics, we selectively express light-sensitive channels in specific projection neurons, then shine a light of a specific wavelength to activate or inhibit ongoing neuronal activity via those channels. (We don’t have published data with this technique yet – stay tuned!)
- With functional MRI (in collaboration with Dr. Ian Shih’s lab), we measure blood oxygenation (BOLD) to determine blood flow, an indirect measure of neural activity, in regions across the brain. By correlating changes in the BOLD signal between regions, we estimate resting-state functional connectivity (Figure 3).
Together these techniques offer a window on information processing in the forebrain while rats engage in motivated behaviors and cognitive tasks. After establishing baseline neuronal activity, we can test the effects of experimental manipulations (homeostatic drives, acute and chronic alcohol or nicotine, therapeutic drugs, alcohol dependence) on the neuronal measurements.
Figure 1. From Stringfield et al., 2017. Orbitofrontal cortical neurons can encode via their firing rate conditioned expectation of reward to a Pavlovian conditioned cue. Rasters and perievent histograms depicting phasic activity of one individual example neuron in the lateral orbitofrontal cortex during a Pavlovian conditioning session. Panels A-F represent individual spikes (above: each tick mark is an action potential and each line is a trial) and averaged firing rate (histogram below, bin size 100 ms)during a 4-s period surrounding an event of interest at time zero (grey bar). Events of interest are noted on each panel, RE = receptacle entry. Note the excitation immediately following receptacle entry when the action occurs during the conditioned cue (B) or at reward retrieval (C), when the same action occurs outside of the cue (A)
. 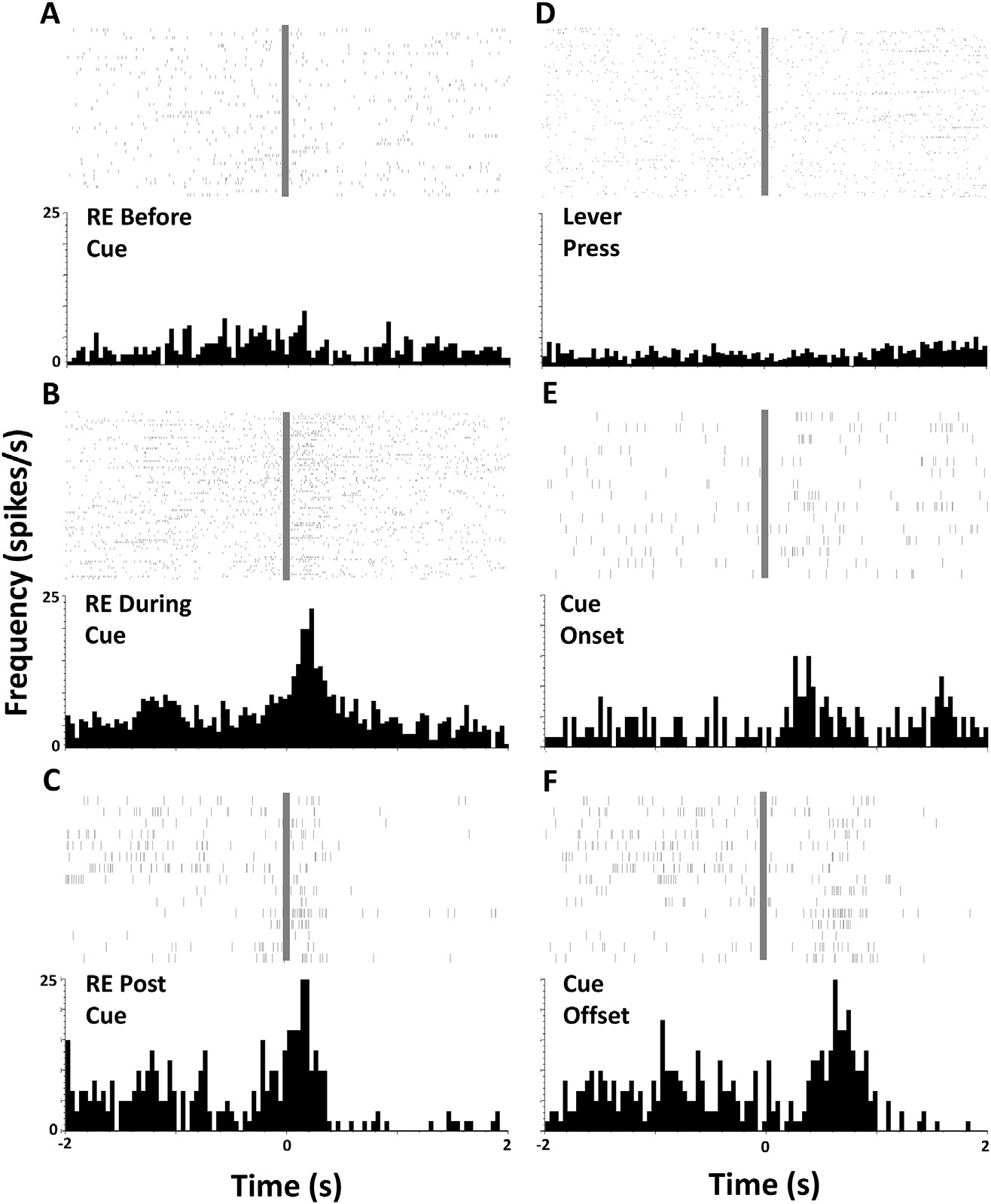
Figure 2. From Shnitko et al., 2016. Dopamine transients occur in the dorsolateral striatum (DLS) at a lever press when it is followed by a reward (reinforced, left) but not when it is unreinforced (right). (A) Representation of phasic dopamine release recorded in the DLS of individual rats. In the color plot, currents resulting from oxidation/reduction on the carbon-fiber surface are depicted in color across applied potentials (y-axis) and time (x-axis); dopamine oxidation occurs at ∼0.65 V. In the traces above the plot, dopamine concentration, converted by principle component regression from the electrochemical signal, is plotted versus time. The time of lever press is indicated by the triangles (white in color plot, black in trace abive). (B) Fluctuations in dopamine concentration in the DLS measured 5 s before and 5 s after reinforced and unreinforced lever presses and averaged across rats within 10S and 10S10E groups (see text for n values). Solids lines indicate mean, and dashed lines indicate SEM. Gray horizontal bars indicate the 2 s period considered as “baseline”. Vertical green dashed lines indicate the time of lever press.
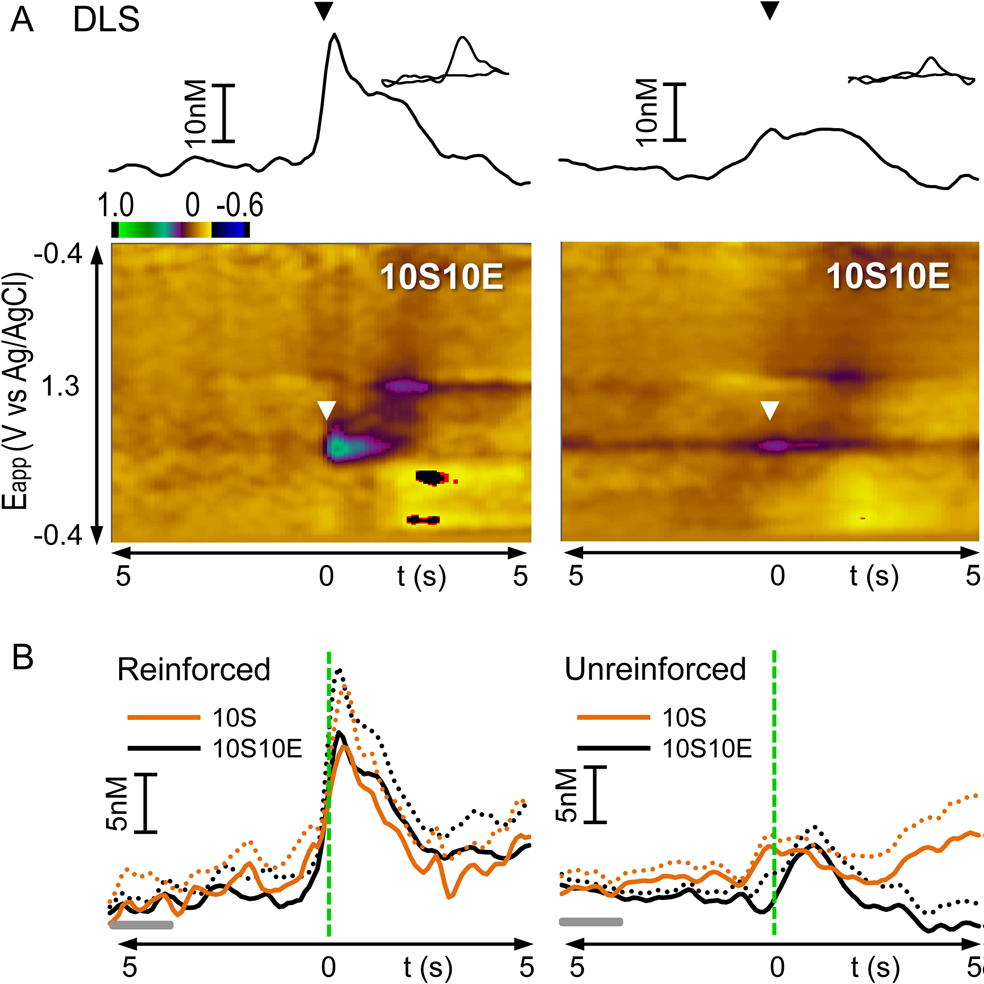
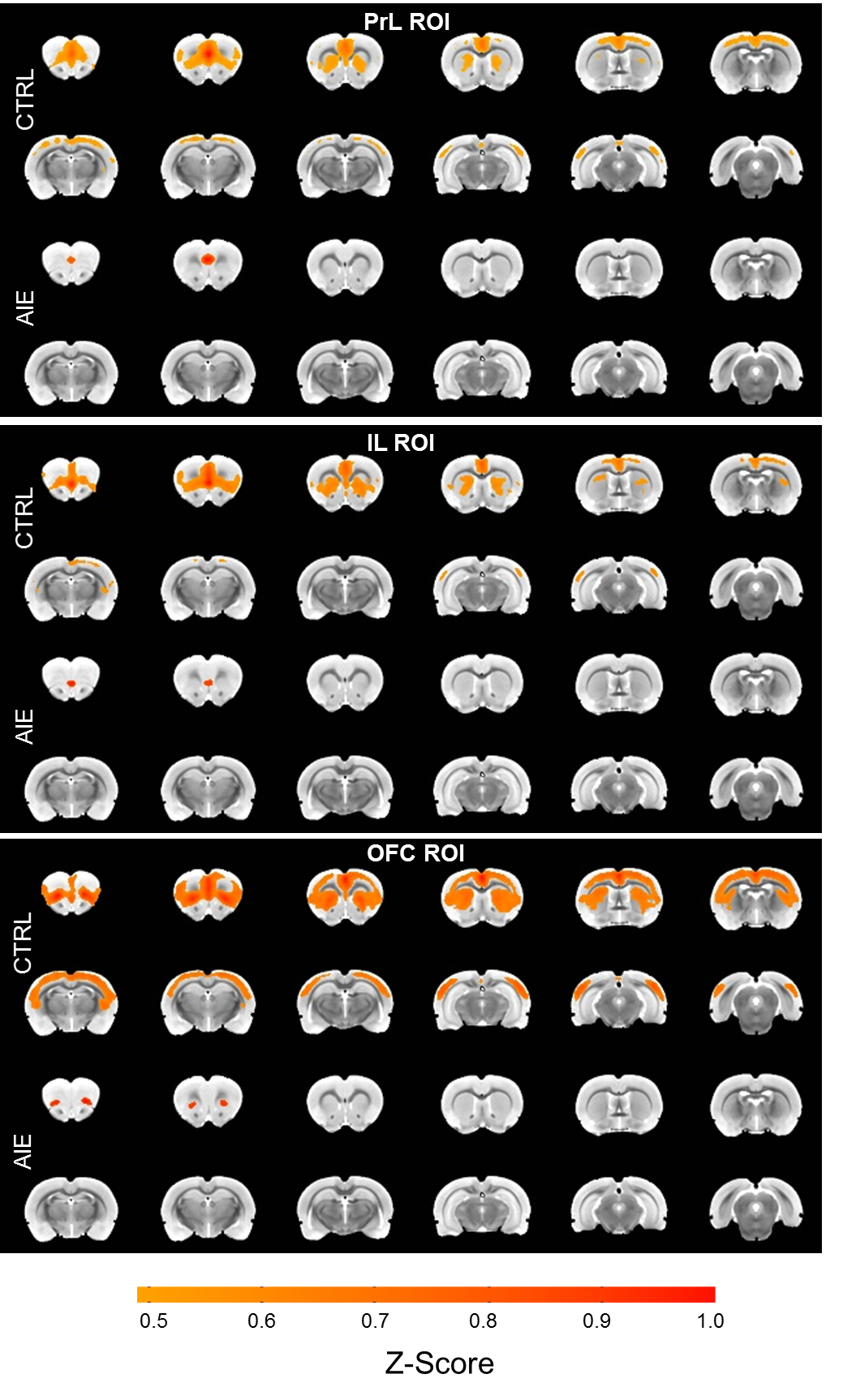
Figure 3. From Broadwater et al., in press. Adolescent intermittent ethanol (AIE) exposure reduces cortico-striatal functional connectivity in adult rats. Voxel-wise correlation maps of baseline data (threshold = 0.5 average Z-score) using the orbitofrontal cortex (OFC) bilateral region-of-interest (ROI) show that control (CTRL) rats have higher levels of connectivity than AIE rats.