
Nicholas Shaheen investigates early detection and treatment of esophageal cancer
Nicholas J. Shaheen, MD, MPH, is the Chief of the Division of Gastroenterology and Hepatology. His group is currently involved in more than 50 research studies.
Dr. Shaheen’s research focuses on the prevention, detection, and treatment of precancerous and cancerous conditions of the esophagus. He is trying to identify clinical and laboratory markers for people at high risk of developing esophageal cancer, and the precursor precancerous condition, Barrett’s esophagus. Currently, the only way to detect these conditions is through endoscopy. A test that could be performed by a primary care doctor might increase early identification of these cancers. Dr. Shaheen’s group is investigating the usefulness of screening with the Cytosponge, a sponge encapsulated in a gelatin capsule (see figure). After swallowing the capsule, the retrievable sponge expands to pick up potentially pre-cancerous cells from the walls of the patient’s esophagus. This device, developed by researchers at Cambridge, UK, holds the promise of cheap, effective, accurate screening for esophageal cancer.
 Another initiative seeks to improve detection of precancerous tissues of the esophagus during endoscopy. To identify more subtle changes associated with cancer, Dr. Shaheen, in conjunction with biomedical engineers at Duke University, places an imaging probe on the end of the endoscope. This probe can image features of precancerous and cancerous tissue, such enlarged nuclei and abnormal architecture, to identify areas of special concern.
Another initiative seeks to improve detection of precancerous tissues of the esophagus during endoscopy. To identify more subtle changes associated with cancer, Dr. Shaheen, in conjunction with biomedical engineers at Duke University, places an imaging probe on the end of the endoscope. This probe can image features of precancerous and cancerous tissue, such enlarged nuclei and abnormal architecture, to identify areas of special concern.
A final research goal is to develop and improve treatment of early esophageal cancer. Just 10 years ago, most patients with early esophageal cancer were treated with surgical removal of the esophagus. In conjunction with other investigators, UNC has led the development of less invasive treatments including killing cancerous areas by endoscopic delivery of either heat or freezing.
Anne Peery and Ian Grimm use a national database to investigate readmissions following surgery for benign colon polyps
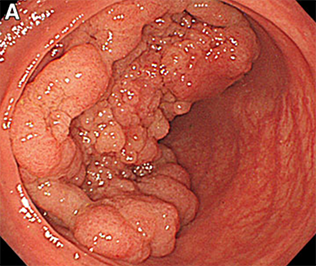 Despite evidence that most non-malignant colorectal polyps can be managed endoscopically, a substantial proportion of patients with a non-malignant colorectal polyp are still sent to surgery. Anne Peery, MD, MSCR and Ian Grimm, MD published a study that found that surgery for a non-malignant colorectal polyp is associated with significant morbidity and mortality. In an analysis of 12,732 patients who underwent elective surgery for a non-malignant colorectal polyp, thirty-day mortality was 0.7% and the risk of a major post-operative adverse event was 14%. This work reinforces the recommendation that patients with non-malignant colorectal polyps should be evaluated for resection by an endoscopist skilled in advanced mucosal resection before consideration of referral for surgery.
Despite evidence that most non-malignant colorectal polyps can be managed endoscopically, a substantial proportion of patients with a non-malignant colorectal polyp are still sent to surgery. Anne Peery, MD, MSCR and Ian Grimm, MD published a study that found that surgery for a non-malignant colorectal polyp is associated with significant morbidity and mortality. In an analysis of 12,732 patients who underwent elective surgery for a non-malignant colorectal polyp, thirty-day mortality was 0.7% and the risk of a major post-operative adverse event was 14%. This work reinforces the recommendation that patients with non-malignant colorectal polyps should be evaluated for resection by an endoscopist skilled in advanced mucosal resection before consideration of referral for surgery.
Dr. Peery was recently awarded a K23 (Mentored Career Development Award) from the NIH to acquire skills in pharmacoepidemiology, comparative effectiveness and patient-reported outcomes research. Her research will generate evidence about the effectiveness, benefits and harms of antibiotics for acute diverticulitis and will lead to a novel diverticulitis specific patient-reported outcomes measure.
Hans Herfarth directs investigator initiated studies in patients with inflammatory bowel diseases (IBD) at the UNC Multidisciplinary Center for IBD Research and Treatment
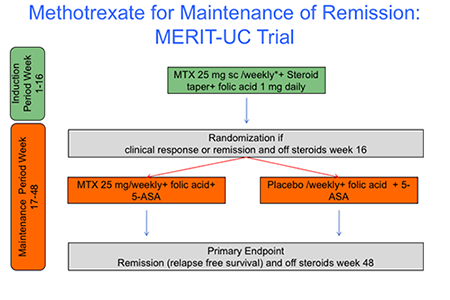 The UNC Multidisciplinary Center for IBD Research and Treatment currently participates in more than 30 clinical studies and registries. One of the strengths of the center is the execution of investigator-initiated trials (IIT’s), facilitated by the established infrastructure at the Center for Gastrointestinal Biology and Disease (CGIBD). Hans Herfarth, MD, is currently the principal investigator of two IIT’s for patients with refractory ulcerative colitis and antibiotic dependent pouchitis. MERIT-UC (MEthotrexate Response In Treatment of Ulcerative Colitis) is a multi-center prospective placebo controlled study to investigate the safety and efficacy of 25 mg MTX in patients with active ulcerative colitis. The MERIT-UC study is the largest NIH-funded IIT in the field of IBD in the last decade Between February 2012 and May 2016, 256 patients were screened and 179 patients were enrolled into the study at 43 sites across the US. The last patient completed the study in April 2017. Currently all data are being analyzed and the results of the trial will be published early 2018.
The UNC Multidisciplinary Center for IBD Research and Treatment currently participates in more than 30 clinical studies and registries. One of the strengths of the center is the execution of investigator-initiated trials (IIT’s), facilitated by the established infrastructure at the Center for Gastrointestinal Biology and Disease (CGIBD). Hans Herfarth, MD, is currently the principal investigator of two IIT’s for patients with refractory ulcerative colitis and antibiotic dependent pouchitis. MERIT-UC (MEthotrexate Response In Treatment of Ulcerative Colitis) is a multi-center prospective placebo controlled study to investigate the safety and efficacy of 25 mg MTX in patients with active ulcerative colitis. The MERIT-UC study is the largest NIH-funded IIT in the field of IBD in the last decade Between February 2012 and May 2016, 256 patients were screened and 179 patients were enrolled into the study at 43 sites across the US. The last patient completed the study in April 2017. Currently all data are being analyzed and the results of the trial will be published early 2018.
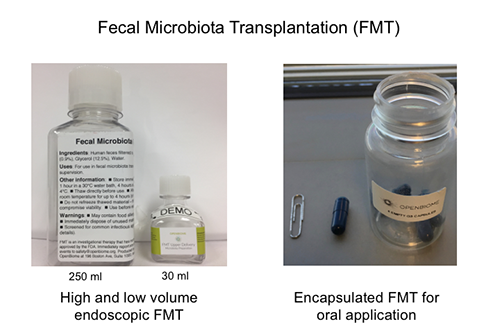 Approximately 20% of patients diagnosed with ulcerative colitis require surgery to remove their colon and construct an ileo-anal pouch anastomosis (IPAA). As a center for surgical therapy in the South East US, more than 2000 pouch procedures have been performed at UNC since the early 1990’s. While pouch surgery is considered curative for ulcerative colitis, 10-20% of the patients with IPAA develop recurrent inflammation of the pouch, termed pouchitis. Chronic antibiotic therapy is helpful in this condition, suggesting a significant role of intestinal bacteria in the pathogenesis of pouchitis. The Broad Crohn’s and Colitis Foundation has recently funded Dr. Herfarth to conduct a pilot study using fecal microbiota transplantation (FMT) in patients with antibiotic dependent pouchitis. The new investigator-initiated study will use a highly innovative approach applying “low volume” endoscopic FMT followed by a 2 weekly daily oral encapsulated FMT to evaluate if patients with antibiotic dependent pouchitis can successfully stop antibiotic therapy.
Approximately 20% of patients diagnosed with ulcerative colitis require surgery to remove their colon and construct an ileo-anal pouch anastomosis (IPAA). As a center for surgical therapy in the South East US, more than 2000 pouch procedures have been performed at UNC since the early 1990’s. While pouch surgery is considered curative for ulcerative colitis, 10-20% of the patients with IPAA develop recurrent inflammation of the pouch, termed pouchitis. Chronic antibiotic therapy is helpful in this condition, suggesting a significant role of intestinal bacteria in the pathogenesis of pouchitis. The Broad Crohn’s and Colitis Foundation has recently funded Dr. Herfarth to conduct a pilot study using fecal microbiota transplantation (FMT) in patients with antibiotic dependent pouchitis. The new investigator-initiated study will use a highly innovative approach applying “low volume” endoscopic FMT followed by a 2 weekly daily oral encapsulated FMT to evaluate if patients with antibiotic dependent pouchitis can successfully stop antibiotic therapy.
Scott Magness has developed a ‘gut on a chip’ to investigate neuroendocrine tumors
Scott Magness, PhD, is a stem cell biologist and geneticist who focuses on GI-tract epithelium and innovative approaches to enhance basic science understanding of stemness for regenerative medicine. His work has a strong translational focus that harnesses stemness to understand cancer initiation and develop strategies to treat chronic inflammatory conditions that impair normal gut regeneration. He holds a multi-PI Transformative R01 along with Drs. Nancy Allbritton, Scott Bultman, and Shawn Gomez to develop a human intestinal ‘simulacrum’, which is a stem cell-driven ‘gut-on-a-chip’ representation of the small intestine and colon made from human patient biopsies. The platform holds strong promise for drug screening, microbiome research, and personalized medicine applications. He has recently been awarded an R21 to investigate neuroendocrine tumors (NETs) of the small intestine. This award was made possible by a pilot study funded by the UNC Lineberger Comprehensive Cancer Center.
NETs are understudied because of their rarity and difficulties in obtaining live tissues to investigate key questions related to cell origin, driver mutations, and microenvironment. Dr. Magness works with Dr. Autumn McRee and the UNC Tissue Procurement Facility to identify candidate patients with GI-NETs for isolation of live tissue that will undergo single-cell RNA-sequencing to map the tumor and microenvironment. The study has a second focus to test candidate driver-mutations by CRISPR-editing of a rare ‘reserve’ intestinal stem cell – a cell that interestingly exhibits properties of stemness and enteroendocrine cells. The study has potential to inform new model of cancer progression and novel treatment methods aimed at unique NET pathways and the microenvironment.
Robert Sandler investigates understudied diseases
 Robert Sandler, MD, MPH is the director of the Center for Gastrointestinal Biology and Disease. After spending most of his career investigating colorectal neoplasms – cancer and polyps – he decided to switch to diseases that are common but have not received much study. He has recently completed a study on diverticulosis, a common condition in the US, funded by the NIH. Diverticula are outpouchings of the lining of the colon through weak areas of the muscle that surrounds the colon. Although diverticula are asymptomatic, complications including bleeding and inflammation (diverticulitis) exceed $4 billion dollars annually.
Robert Sandler, MD, MPH is the director of the Center for Gastrointestinal Biology and Disease. After spending most of his career investigating colorectal neoplasms – cancer and polyps – he decided to switch to diseases that are common but have not received much study. He has recently completed a study on diverticulosis, a common condition in the US, funded by the NIH. Diverticula are outpouchings of the lining of the colon through weak areas of the muscle that surrounds the colon. Although diverticula are asymptomatic, complications including bleeding and inflammation (diverticulitis) exceed $4 billion dollars annually.
A series of papers written with Anne Peery, MD, MSCR, suggested that most of what people previously believed about diverticulosis was probably wrong. Several papers cast doubt on the widely held belief that a high fiber protected against diverticulosis. Also contrary to expectation, neither constipation nor physical inactivity were associated with diverticulosis. We found no association between diverticula and colonic neoplasia and presented data to refute the idea that colonic diverticulosis is associated with low-grade chronic mucosal inflammation.
Microscopic colitis is a condition where the appearance of the colon is normal, but biopsies show microscopic inflammation – thus the name. Microscopic colitis is now the most common cause of watery diarrhea in the elderly. Dr. Sandler currently has the only NIH R01 grant on this topic. The project, currently in its first year, is enrolling patients with diarrhea to learn more about risk factors, severity and biological features.
Michael Fried directs HCV-TARGET, an observational study of patients with hepatitis C to gain real-world evidence of the safety and efficacy of therapies
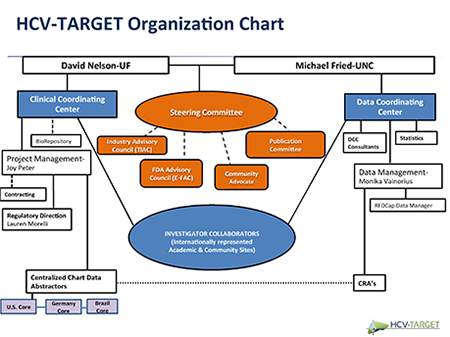 HCV-TARGET, a collaborative platform with academic thought leaders, regulatory agencies, pharmaceutical sponsors, and patient advocacy groups, is performing a longitudinal, observational study of patients with chronic hepatitis C (Figure) under the direction of Michael W. Fried, MD. The aim of HCV-TARGET (NCT01474811) is to develop real-world evidence to evaluate the safety and effectiveness of HCV therapies used in usual clinical practice, to improve understanding of therapeutic response in populations underrepresented in phase 3 trials, and to provide well-characterized biospecimens for translational studies. The consortium includes academic (n=44) and community (n=17) centers from North America and Europe (n=4).
HCV-TARGET, a collaborative platform with academic thought leaders, regulatory agencies, pharmaceutical sponsors, and patient advocacy groups, is performing a longitudinal, observational study of patients with chronic hepatitis C (Figure) under the direction of Michael W. Fried, MD. The aim of HCV-TARGET (NCT01474811) is to develop real-world evidence to evaluate the safety and effectiveness of HCV therapies used in usual clinical practice, to improve understanding of therapeutic response in populations underrepresented in phase 3 trials, and to provide well-characterized biospecimens for translational studies. The consortium includes academic (n=44) and community (n=17) centers from North America and Europe (n=4).
Prospective data (Demographics, comorbidities, conmeds, clinical, adverse events, and virologic data) are collected through treatment and follow-up from sequentially enrolled, consented patients. Data are entered into a common REDCAP database using standardized and novel, source data abstraction. A centralized team of trained coders reviews all redacted medical records (narratives, laboratory data, imaging reports) obtained from clinical sites who are compensated for participation. The team systematically monitors the data for completeness and accuracy.
Over 11,000 patients have been consented and enrolled in the ongoing HCV-TARGET study. To date, HCV-TARGET has developed over 25 presentations at national and international meetings with 10 manuscripts published in high-impact journals. Importantly, the evidence generated from HCV-TARGET has informed HCV treatment guidelines from AASLD/IDSA and the World Health Organization (WHO). Pharmaceutical collaborators have utilized HCV-TARGET data to support label expansion and to meet post-marketing requirements. The unique HCV-TARGET model is currently serving as the platform for a randomized, pragmatic clinical trial of HCV therapeutics (PRIORITIZE, NCT02601820), and for a study of patient-reported outcomes (PROP-UP, NCT02601820), both funded by PCORI. The TARGET model is also being applied to other disease areas, including TARGET-NASH, TARGET-HCC, TARGET-PBC, and TARGET-IBD).
Evan Dellon optimizing esophageal drug delivery in eosinophilic esophagitis
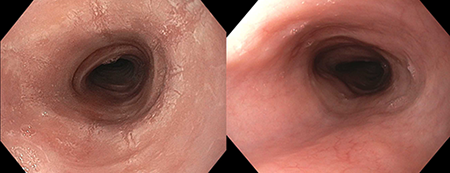 Eosinophilic esophagitis (EoE) is a chronic inflammatory condition of the esophagus where eosinophils infiltrate the esophageal mucosa and lead to esophageal narrowing and strictures. Clinically, particularly in adolescents and adults, this manifests as dysphagia and food impaction. Treatment of EoE can be challenging, in large part because there are currently no FDA-approved medications. The main pharmacologic treatment is off-label use of corticosteroids. With this approach, an asthma preparation such as a multi-dose inhaler of fluticasone or an aqueous nebulizer solution of budesonide, is swallowed rather than inhaled to coat the esophagus. As would be expected with a “homemade” approach like this, response is quite variable and non-response is common. An excellent response, as illustrated by improvement in the appearance of the esophagus, is shown in the figure with the appearance before treatment on the left and after treatment on the right.
Eosinophilic esophagitis (EoE) is a chronic inflammatory condition of the esophagus where eosinophils infiltrate the esophageal mucosa and lead to esophageal narrowing and strictures. Clinically, particularly in adolescents and adults, this manifests as dysphagia and food impaction. Treatment of EoE can be challenging, in large part because there are currently no FDA-approved medications. The main pharmacologic treatment is off-label use of corticosteroids. With this approach, an asthma preparation such as a multi-dose inhaler of fluticasone or an aqueous nebulizer solution of budesonide, is swallowed rather than inhaled to coat the esophagus. As would be expected with a “homemade” approach like this, response is quite variable and non-response is common. An excellent response, as illustrated by improvement in the appearance of the esophagus, is shown in the figure with the appearance before treatment on the left and after treatment on the right.
A major focus of research by Evan Dellon, MD, MPH, is to help identify better methods of delivering medications directly to the esophagus. Several years ago, his research group conducted a small randomized controlled trial of two different formulations of budesonide, and found that histologic improvement in patients with EoE was directly proportional to how long the medication was able to “dwell” in the esophagus. They are now conducting a much larger randomized study, which is funded by NIH, comparing the efficacy of a budesonide formulation to that of a fluticasone formulation, with the goal of establishing the most appropriate first line swallowed/topical steroid for use in EoE. In addition, there are several ongoing industry-sponsored studies looking at novel esophageal-specific medication formulations for EoE, and the UNC Center for Esophageal Diseases and Swallowing (CEDAS) is a site for these trials, all of which are actively enrolling (contact eoe@unc.edu for more information). Finally, working with colleagues at the NC State College of Veterinary Medicine and the UNC Eshelman School of Pharmacy, and using the large animal core of the UNC Center for Gastrointestinal Biology and Disease, the investigators are establishing novel models and techniques to assess and optimize esophageal drug delivery.
Other research news
Between May 1, 2016 and April 30, 2017 members of the GI division received $19,252,933 in external grant support. There were 21 faculty members in the division who received grant support from the federal government, foundations, and industry. There were more than $7.7 million from the NIH including two T32 training grants, a Digestive Disease Center Grant and grants of several other types.
A recently performed analysis of research funding in University GI divisions was presented at DDW in May. This analysis demonstrated that only 25% of divisions had 2 or more NIH funded investigators. UNC has11 NIH funded investigators. The poster also ranked US GI divisions by research dollars. The UNC GI division was among the leaders nationally in NIH funding to GI divisions.