Specialty Areas:
Pathophysiology of airway mucociliary clearance in health and disease, biology of human small airways.
Chronology:
Dr. Okuda obtained his M.D. degree from Yamagata University in Japan (2010), followed by residency training in internal medicine and fellowship in respiratory medicine. During the fellowship, he worked at Tokyo National Hospital, which was a 300-bed hospital for patients with respiratory diseases. Thereafter, he entered the graduate school of medicine at the University of Tokyo in Japan. In the second year of graduate school, he applied for postdoctoral training in Dr. Richard Boucher’s laboratory at The University of North Carolina (UNC) at Chapel Hill to engage in studies of airway and mucus biology. Using the study conducted at UNC at Chapel Hill, he earned his Ph.D. degree in medicine from The University of Tokyo (2019). After a 2-year period as a Research Associate, Dr. Okuda was promoted in 2021 to an Assistant Professor in the Department of Medicine, Division of Pulmonary Diseases and Critical Care Medicine at UNC at Chapel Hill.
Research Focus:
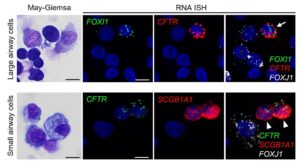
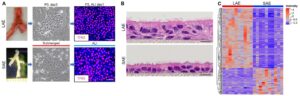
Dr. Okuda’s overall research interest focuses on how the mucociliary clearance (MCC) system is regulated to maintain homeostasis in the lung and how it fails in muco-obstructive lung diseases, including cystic fibrosis (CF), asthma, and COPD. During the training period under Dr. Richard Boucher’s supervision, Dr. Okuda successfully characterized the regional expression patterns of major airway secretory mucins, MUC5AC/MUC5B, and CFTR/ionocytes in normal and CF human airways (Am J Respir Crit Care Med, 2019, 2021). These investigations provide the reagents and techniques for his career. Dr. Okuda has extensive experience in handling single cells obtained from in vivo and in vitro materials for single cell-based transcriptomic analysis. Further, he has developed microdissection techniques for human small airways and in vitro and explant small airway epithelial cell cultures to study small airway-specific biology. Also, given the emergent situation caused by SARS-CoV-2 pandemic, Dr. Okuda has been actively engaged in collaborations with Dr. Ralph Baric lab at UNC to utilize his skills and materials for COVID-19 studies. Based on these research interests and experience, Dr. Okuda’s long-term career goal is to work, as a professional physician-scientist, toward a full understanding of the MCC system in the lung and contribute to the improvement of the prognosis in all patients with muco-obstructive lung diseases.

Selected Bibliography:
- Asakura T, Okuda K, Chen G, Dang H, Kato T, Mikami Y, Schworer SA, Gilmore RC, Radicioni G, Hawkins P, Barbosa Cardenas SM, Saito M, Cawley AM, De la Cruz G, Chua M, Alexis NE, Masugi Y, Noone PG, Ribeiro CMP, Kesimer M, Olivier KN, Hasegawa N, Randell SH, O’Neal WK, Boucher RC. Proximal and Distal Bronchioles Contribute to the Pathogenesis of Non-Cystic Fibrosis Bronchiectasis (NCFB). Am J Respir Crit Care Med. 2024 Feb 15;209(4):374-389. doi: 10.1164/rccm.202306-1093OC. PMID: 38016030. PMCID: PMC10878387.
- He Z, Chu C, Dickson R, Okuda K, Cai L. A gel-coated air-liquid-interface culture system with tunable substrate stiffness matching healthy and diseased lung tissues. Am J Physiol Lung Cell Mol Physiol. 2024 Jan 22. doi: 10.1152/ajplung.00153.2023. Epub ahead of print. PMID: 38252871.
- Nakakubo S*, Kishida N*, Okuda K*, Kamada K, Iwama M, Suzuki M, Yokota I, Ito YM, Nasuhara Y, Boucher RC, Konno S. Associations of COVID-19 symptoms with omicron subvariants BA.2 and BA.5, host status, and clinical outcomes in Japan: A registry-based observational study. Lancet Infect Dis. 2023 Nov;23(11):1244-1256. doi: 10.1016/S1473-3099(23)00271-2. PMID: 37399831. PMCID: PMC10615696. *These authors equally contributed to the work.
- Leach T, Gandhi U, Reeves KD, Stumpf K, Okuda K, Marini FC, Walker SJ, Boucher R, Chan J, Cox LA, Atala A, Murphy SV. Development of a novel air-liquid interface airway tissue equivalent model for in vitro respiratory modeling studies. Sci Rep. 2023 Jun 22;13(1):10137. doi: 10.1038/s41598-023-36863-1. PMID: 37349353; PMCID: PMC10287689.
- Mikami Y, Grubb BR, Rogers TD, Dang H, Asakura T, Kota P, Gilmore RC, Okuda K, Morton LC, Sun L, Chen G, Wykoff JA, Ehre C, Vilar J, van Heusden C, Livraghi-Butrico A, Gentzsch M, Button B, Stutts MJ, Randell SH, O’Neal WK, Boucher RC. Chronic airway epithelial hypoxia exacerbates injury in muco-obstructive lung disease through mucus hyperconcentration. Sci Transl Med. 2023 Jun 7;15(699):eabo7728. doi: 10.1126/scitranslmed.abo7728. PMID: 37285404. PMCID: PMC10664029.
- Barnett KC, Xie Y, Asakura T, Song D, Liang K, Taft-Benz SA, Guo H, Yang S, Okuda K, Gilmore RC, Loome JF, Oguin Iii TH, Sempowski GD, Randell SH, Heise MT, Lei YL, Boucher RC, Ting JP. An epithelial-immune circuit amplifies inflammasome and IL-6 responses to SARS-CoV-2. Cell Host Microbe. 2023 Feb 8;31(2):243-259.e6. doi: 10.1016/j.chom.2022.12.005. PMID: 36563691; PMCID: PMC9731922.
- Kato T, Asakura T, Edwards CE, Dang H, Mikami Y, Okuda K, Chen G, Sun L, Gilmore RC, Hawkins P, De la Cruz G, Cooley MR, Bailey AB, Hewitt SM, Chertow DS, Borczuk AC, Salvatore S, Martinez FJ, Thorne LB, Askin FB, Ehre C, Randell SH, O’Neal WK, Baric RS, Boucher RC; NIH COVID-19 Autopsy Consortium. Prevalence and mechanisms of mucus accumulation in COVID-19 lung disease. Am J Respir Crit Care Med. 2022 Dec 1;206(11):1336-1352. doi: 10.1164/rccm.202111-2606OC. PMID: 35816430. PMCID: PMC9746856.
- Dinnon KH§, Leist SR§, Okuda K§, Dang H§, Fritch EJ§, Gully KL, De la Cruz G, Evangelista MD, Asakura T, Gilmore RC, Hawkins P, Nakano S, West A, Schäfer A, Gralinski LE, Everman JL, Sajuthi SP, Zweigart MR, Dong S, McBride J, Cooley MR, Hines JB, Love MK, Groshong SD, VanSchoiack A, Phelan SJ, Liang Y, Hether T, Leon M, Zumwalt RE, Barton LM, Duval EJ, Mukhopadhyay S, Stroberg E, Borczuk A, Thorne LB, Sakthivel MK, Lee YZ, Hagood JS, Mock JR, Seibold MA, O’Neal WK, Montgomery SA, Boucher RC*, Baric RS*. SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci Transl Med. 2022 Sep 28;14(664):eabo5070. doi: 10.1126/scitranslmed.abo5070. PMID: 35857635. PMCID: PMC9273046. §These authors contributed equally. *Joint senior authorship.
- Okuda K, Shaffer KM, Ehre C. Mucins and CFTR: Their Close Relationship. Int J Mol Sci. 2022 Sep 6;23(18):10232. doi: 10.3390/ijms231810232. PMID: 36142171. PMCID: PMC9499620.
- Schäfer A, Leist SR, Gralinski LE, Martinez DR, Winkler ES, Okuda K, Hawkins PE, Gully KL, Graham RL, Scobey DT, Bell TA, Hock P, Shaw GD, Loome JF, Madden EA, Anderson E, Baxter VK, Taft-Benz SA, Zweigart MR, May SR, Dong S, Clark M, Miller DR, Lynch RM, Heise MT, Tisch R, Boucher RC, Pardo Manuel de Villena F, Montgomery SA, Diamond MS, Ferris MT, Baric RS. A multitrait locus regulates sarbecovirus pathogenesis. mBio. 2022 Aug 30;13(4):e0145422. doi: 10.1128/mbio.01454-22. PMID: 35862771. PMCID: PMC9426612.
- Biering SB, Sarnik SA, Wang E, Zengel JR, Leist SR, Schäfer A, Sathyan V, Hawkins P, Okuda K, Tau C, Jangid AR, Duffy CV, Wei J, Gilmore RC, Alfajaro MM, Strine MS, Nguyenla X, Van Dis E, Catamura C, Yamashiro LH, Belk JA, Begeman A, Stark JC, Shon DJ, Fox DM, Ezzatpour S, Huang E, Olegario N, Rustagi A, Volmer AS, Livraghi-Butrico A, Wehri E, Behringer RR, Cheon DJ, Schaletzky J, Aguilar HC, Puschnik AS, Button B, Pinsky BA, Blish CA, Baric RS, O’Neal WK, Bertozzi CR, Wilen CB, Boucher RC, Carette JE, Stanley SA, Harris E, Konermann S, Hsu PD. Genome-wide bidirectional CRISPR screens identify mucins as host factors modulating SARS-CoV-2 infection. Nat Genet. 2022 Aug;54(8):1078-1089. doi: 10.1038/s41588-022-01131-x. PMID: 35879412. PMCID: PMC9355872.
- Kadur Lakshminarasimha Murthy P, Sontake V, Tata A, Kobayashi Y, Macadlo L, Okuda K, Conchola AS, Nakano S, Gregory S, Miller LA, Spence JR, Engelhardt JF, Boucher RC, Rock JR, Randell SH, Tata PR. Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature. 2022 Apr;604(7904):111-119. doi: 10.1038/s41586-022-04541-3. PMID: 35355018. PMCID: PMC9169066.
- Kato T, Radicioni G, Papanikolas MJ, Stoychev GV, Markovetz MR, Aoki K, Porterfield M, Okuda K, Barbosa Cardenas SM, Gilmore RC, Morrison CB, Ehre C, Burns KA, White KK, Brennan TA, Goodell HP, Thacker H, Loznev HT, Forsberg LJ, Nagase T, Rubinstein M, Randell SH, Tiemeyer M, Hill DB, Kesimer M, O’Neal WK, Ballard ST, Freeman R, Button B, Boucher RC. Mucus concentration-dependent biophysical abnormalities unify submucosal gland and superficial airway dysfunction in cystic fibrosis. Sci Adv. 2022 Apr;8(13):eabm9718. doi: 10.1126/sciadv.abm9718. PMID: 35363522.
- McElvaney OF, Asakura T, Meinig SL, Torres-Castillo JL, Hagan RS, Gabillard-Lefort C, Murphy MP, Thorne LB, Borczuk A, Reeves EP, Zumwalt RE, Mikami Y, Carroll TP, Okuda K, Hogan G, McElvaney OJ, Clarke J, McEvoy NL, Mallon PW, McCarthy C, Curley G, Wolfgang MC, Boucher RC, McElvaney NG. Protease-anti-protease compartmentalization in SARS-CoV-2 ARDS: Therapeutic implications. EBioMedicine. 2022 Mar;77:103894. doi: 10.1016/j.ebiom.2022.103894. PMID: 35217407; PMCID: PMC8861575.
- Okuda K, Randell SH, Birket SE. The Big Impact of Small Airway pH. Am J Respir Cell Mol Biol. 2021 Aug;65(2):123-125. doi: 10.1165/rcmb.2021-0070ED. PMID: 33831321; PMCID: PMC8399579.
- Okuda K, Dang H, Kobayashi Y, Carraro G, Nakano S, Chen G, Kato T, Asakura T, Gilmore RC, Morton LC, Lee RE, Mascenik T, Yin WN, Barbosa Cardenas SM, O’Neal YK, Minnick CE, Chua M, Quinney NL, Gentzsch M, Anderson CW, Ghio A, Matsui H, Nagase T, Ostrowski LE, Grubb BR, Olsen JC, Randell SH, Stripp BR, Tata PR, O’Neal WK, Boucher RC. Secretory Cells Dominate Airway CFTR Expression and Function in Human Airway Superficial Epithelia. Am J Respir Crit Care Med. 2021 May 15;203(10):1275-1289. doi: 10.1164/rccm.202008-3198OC. PMID: 33321047. PMCID: PMC8456462.
- Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, Pelayo E, Maldonado JO, Lafont BA, Jang SI, Nasir N, Padilla RJ, Murrah VA, Maile R, Lovell W, Wallet SM, Bowman NM, Meinig SL, Wolfgang MC, Choudhury SN, Novotny M, Aevermann BD, Scheuermann RH, Cannon G, Anderson CW, Lee RE, Marchesan JT, Bush M, Freire M, Kimple AJ, Herr DL, Rabin J, Grazioli A, Das S, French BN, Pranzatelli T, Chiorini JA, Kleiner DE, Pittaluga S, Hewitt SM, Burbelo PD, Chertow D; NIH COVID-19 Autopsy Consortium; HCA Oral and Craniofacial Biological Network, Frank K, Lee J, Boucher RC, Teichmann SA, Warner BM, Byrd KM. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021 May;27(5):892-903. doi: 10.1038/s41591-021-01296-8. PMID: 33767405. PMCID: PMC8240394.
- Hou YJ, Chiba S, Halfmann P, Ehre C, Kuroda M, Dinnon KH 3rd, Leist SR, Schäfer A, Nakajima N, Takahashi K, Lee RE, Mascenik TM, Graham R, Edwards CE, Tse LV, Okuda K, Markmann AJ, Bartelt L, de Silva A, Margolis DM, Boucher RC, Randell SH, Suzuki T, Gralinski LE, Kawaoka Y, Baric RS. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020 Dec 18;370(6523):1464-1468. doi: 10.1126/science.abe8499. PMID: 33184236. PMCID: PMC7775736.
- Miyashita N, Horie M, Suzuki HI, Saito M, Mikami Y, Okuda K, Boucher RC, Suzukawa M, Hebisawa A, Saito A, Nagase T. FOXL1 Regulates Lung Fibroblast Function via Multiple Mechanisms. Am J Respir Cell Mol Biol. 2020 Dec;63(6):831-842. doi: 10.1165/rcmb.2019-0396OC. PMID: 32946266. PMCID: PMC8017595.
- Leist SR, Dinnon KH 3rd, Schäfer A, Tse LV, Okuda K, Hou YJ, West A, Edwards CE, Sanders W, Fritch EJ, Gully KL, Scobey T, Brown AJ, Sheahan TP, Moorman NJ, Boucher RC, Gralinski LE, Montgomery SA, Baric RS. A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell. 2020 Sep 23:S0092-8674(20)31247-2. doi: 10.1016/j.cell.2020.09.050. PMID: 33031744; PMCID: PMC7510428.
- Hou YJ*, Okuda K*, Edwards CE*, Martinez DR*, Asakura T, Dinnon III KH, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Fulcher L, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O’Neal WK, Randell SH, Boucher RC**, Baric RS**. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020 Jul 23;182(2):429-446.e14. doi: 10.1016/j.cell.2020.05.042. PMID: 32526206; PMCID: PMC7250779. *Co-first authors. **Co-senior authors.
- Rao W, Wang S, Mahalingam R, Duleba M, Xie J, Gollar K, Niroula S, Qi Y, Neupane R, Liew A, Vincent M, Okuda K, O’Neal WK, Boucher RC, Dickey BF, Wechsler ME, Ibrahim O, Engelhardt JF, Crum CP, Mertens TCJ, Wang W, Jyothula SSK, Karmouty-Quintana H, Parekh KR, Metersky ML, McKeon FD, Xian W. Regenerative metaplastic clones in COPD lung drive inflammation and fibrosis. Cell. 2020 May 14;181(4):848-864.e18. doi: 10.1016/j.cell.2020.03.047. PMID: 32298651. PMCID: PMC7294989.
- Chen G, Sun L, Kato T, Okuda K, Martino MB, Abzhanova A, Lin JM, Gilmore RC, Batson BD, Volmer AS, Dang H, Deng Y, Randell SH, Button B, Livraghi-Butrico A, Kesimer M, Ribeiro CMP, O’Neal WK, Boucher RC. IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J Clin Invest. 2019 Oct 1;129(10):4433-4450. doi: 10.1172/JCI125669. PMID: 31524632. PMCID: PMC6763234.
- Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, Martino MB, Dang H, Abzhanova A, Lin JM, Hull-Ryde EA, Volmer AS, Randell SH, Livraghi-Butrico A, Deng Y, Scherer PE, Stripp BR, O’Neal WK, Boucher RC. XBP1S regulates MUC5B in a promoter variant-dependent pathway in IPF airway epithelia. Am J Respir Crit Care Med. 2019 Jul 15;200(2):220-234. doi: 10.1164/rccm.201810-1972OC. PMID: 30973754. PMCID: PMC6635783.
- Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, Radicioni G, Kesimer M, Chua M, Livraghi-Butrico A, Ehre C, Doerschuk CM, Randell SH, Matsui H, Nagase T, O’Neal WK, Boucher RC. Localization of secretory mucins MUC5AC and MUC5B in normal human airways. Am J Respir Crit Care Med. 2019 Mar 15;199(6):715-727. doi: 10.1164/rccm.201804-0734OC. PMID: 30352166. PMCID: PMC6423099.
- Ando T, Kawashima M, Masuda K, Takeda K, Okuda K, Suzuki J, Ohshima N, Horibe M, Tamura A, Nagai H, Matsui H, Ohta K. Exacerbation of chronic pulmonary aspergillosis was associated with a high rebleeding rate after bronchial artery embolization. Respir Investig. 2019 May;57(3):260-267. doi: 10.1016/j.resinv.2018.12.009. PMID: 30692051.
- Kato T, Kobayashi K, Suzukawa M, Saito M, Okuda K, Koyama K, Igarashi S, Arakawa S, Ohshima N, Matsui H, Nagase T, Ohta K. Epithelial-mesenchymal transition of human lung adenocarcinoma A549 cells up-regulates cytokine production upon LPS stimulation. Allergol Int. 2017 Sep;66S:S56-S58. doi: 10.1016/j.alit.2017.06.014. PMID: 28712741.
- Ando T, Kawashima M, Masuda K, Takeda K, Okuda K, Suzuki J, Ohshima N, Matsui H, Tamura A, Nagai H, Akagawa S, Ohta K. Clinical and Angiographic Characteristics of 35 Patients With Cryptogenic Hemoptysis. Chest. 2017 Nov;152(5):1008-1014. doi: 10.1016/j.chest.2017.05.007. PMID: 28526654.
- Okuda K, Masuda K, Kawashima M, Ando T, Koyama K, Ohshima N, Tamura A, Nagai H, Akagawa S, Matsui H, Ohta K. Bronchial artery embolization to control hemoptysis in patients with Mycobacterium avium complex. Respir Investig. 2016 Jan;54(1):50-8. doi: 10.1016/j.resinv.2015.08.004. PMID: 26718145.
- Koyama K, Ohshima N, Suzuki J, Kawashima M, Okuda K, Sato R, Suzukawa M, Nagai H, Matsui H, Ohta K. Evaluation of clinical characteristics and prognosis of chronic pulmonary aspergillosis depending on the underlying lung diseases: Emphysema vs prior tuberculosis. J Infect Chemother. 2015 Nov;21(11):795-801. doi: 10.1016/j.jiac.2015.08.006. PMID: 26410549.
- Koyama K, Ohshima N, Kawashima M, Okuda K, Sato R, Nagai H, Matsui H, Ohta K. Characteristics of pulmonary Mycobacterium avium complex disease diagnosed later in follow-up after negative mycobacterial study including bronchoscopy. Respir Med. 2015 Oct;109(10):1347-53. doi: 10.1016/j.rmed.2015.08.016. PMID: 26365483.
A full list of Kenichi Okuda’s publication can be found at the following link: https://www.ncbi.nlm.nih.gov/myncbi/1fg8udpEgVZE1k/bibliography/public/
