Specialty Areas:
Structure and Function of Cilia; Diseases of Cilia and Mucociliary Clearance; Gene Therapy of CF and Primary Ciliary Dyskinesia
Research Focus:
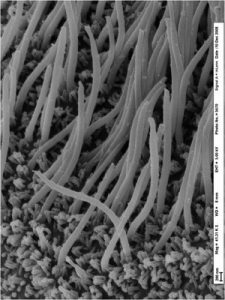
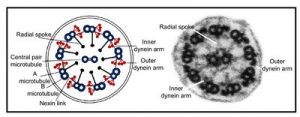
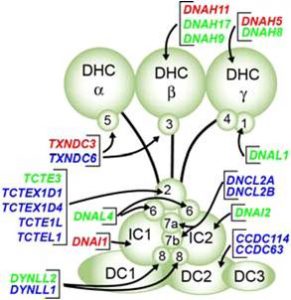
The overall focus of research in my laboratory is to improve the diagnosis and treatment of airway diseases, especially those that result from impaired mucociliary clearance. Mucociliary clearance is the process by which inhaled pathogens and particulate matter are swept out of the lungs by the coordinated beating of the cilia that line the airways (Figure 1). In particular, our efforts focus on the diseases cystic fibrosis and primary ciliary dyskinesia, two inherited diseases caused by mutations that impair mucociliary clearance and lead to recurrent lung infections. The work in our laboratory ranges from basic studies of ciliated cells and the proteins that make up the complex structure of the motile cilia (Figure 2), to translational studies of new drugs and gene therapy vectors. Our laboratory uses a number of model systems, including traditional and inducible animal models, in vitro culture of differentiated mouse and human airway epithelial cells, and direct studies of human tissues. We also use a wide range of experimental techniques, from studies of RNA expression and proteomics to measuring ciliary activity in cultured cells and whole animals.
Selected Bibliography:
- Sears PR, Ostrowski LE. Mucociliary Transport Device Construction and Application to Study Mucociliary Clearance. Methods Mol Biol. 2024;2725:263-276. doi: 10.1007/978-1-0716-3507-0_17. PMID: 37856031.
- Yin W, Golliher HL, Ferguson AJ, Kimbell JS, Livraghi-Butrico A, Rogers TD, Grubb BR, Kimple AJ, Ostrowski LE. Mucolytic treatment of chronic rhinosinusitis in a murine model of primary ciliary dyskinesia. Front Mol Biosci. 2023 Jul 24;10:1221796. doi: 10.3389/fmolb.2023.1221796. PMID: 37555015; PMCID: PMC10405821.
- Smith AJ, Bustamante-Marin XM, Yin W, Sears PR, Herring LE, Dicheva NN, López-Giráldez F, Mane S, Tarran R, Leigh MW, Knowles MR, Zariwala MA, Ostrowski LE. The role of SPAG1 in the assembly of axonemal dyneins in human airway epithelia. J Cell Sci. 2022 Mar 15;135(6):jcs259512. doi: 10.1242/jcs.259512. PMID: 35178554. PMCID: PMC8995097.
- Rogers TD, Button B, Kelada SNP, Ostrowski LE, Livraghi-Butrico A, Gutay MI, Esther CR Jr, Grubb BR. Regional Differences in Mucociliary Clearance in the Upper and Lower Airways. Front Physiol. 2022 Mar 9;13:842592. doi: 10.3389/fphys.2022.842592. PMID: 35356083; PMCID: PMC8959816.
- Ostrowski LE, Yin W, Smith AJ, Sears PR, Bustamante-Marin XM, Dang H, Hildebrandt F, Daniels LA, Capps NA, Sullivan KM, Leigh MW, Zariwala MA, Knowles MR. Expression of a Truncated Form of ODAD1 Associated with an Unusually Mild Primary Ciliary Dyskinesia Phenotype. Int J Mol Sci. 2022 Feb 3;23(3):1753. doi: 10.3390/ijms23031753. PMID: 35163670; PMCID: PMC8835943.
- Kato T, Mikami Y, Sun L, Rogers TD, Grubb BR, Morrison CB, Ehre C, Sears PR, Ostrowski LE, Randell SH, Boucher RC. Reuse of Cell Culture Inserts for in vitro Human Primary Airway Epithelial Cell Studies. Am J Respir Cell Mol Biol. 2021 Jun;64(6):760-764. doi: 10.1165/rcmb.2021-0033LE. PMID: 33788673. PMCID: PMC8456889.
- Zhao Y, Pinskey J, Lin J, Yin W, Sears PR, Daniels LA, Zariwala MA, Knowles MR, Ostrowski LE, Nicastro D. Structural insights into the cause of human RSPH4A primary ciliary dyskinesia. Mol Biol Cell. 2021 Jun 1;32(12):1202-1209. doi: 10.1091/mbc.E20-12-0806. PMID: 33852348. PMCID: PMC8351563.
- Okuda K, Dang H, Kobayashi Y, Carraro G, Nakano S, Chen G, Kato T, Asakura T, Gilmore RC, Morton LC, Lee RE, Mascenik T, Yin WN, Barbosa Cardenas SM, O’Neal YK, Minnick CE, Chua M, Quinney NL, Gentzsch M, Anderson CW, Ghio A, Matsui H, Nagase T, Ostrowski LE, Grubb BR, Olsen JC, Randell SH, Stripp BR, Tata PR, O’Neal WK, Boucher RC. Secretory Cells Dominate Airway CFTR Expression and Function in Human Airway Superficial Epithelia. Am J Respir Crit Care Med. 2021 May 15;203(10):1275-1289. doi: 10.1164/rccm.202008-3198OC. PMID: 33321047. PMCID: PMC8456462.
- Sears PR, Bustamante-Marin XM, Gong H, Markovetz MR, Superfine R, Hill DB, Ostrowski LE. Induction of ciliary orientation by matrix patterning and characterization of mucociliary transport. Biophys J. 2021 Apr 20;120(8):1387-1395. doi: 10.1016/j.bpj.2021.01.041. PMID: 33705757. PMCID: PMC8105732.
- Lee L, Ostrowski LE. Motile cilia genetics and cell biology: big results from little mice. Cell Mol Life Sci. 2021 Feb;78(3):769-797. doi: 10.1007/s00018-020-03633-5. PMID: 32915243. PMCID: PMC7902362.
- Bustamante-Marin XM, Shapiro A, Sears PR, Charng WL, Conrad DF, Leigh MW, Knowles MR, Ostrowski LE, Zariwala MA. Identification of genetic variants in CFAP221 as a cause of primary ciliary dyskinesia. J Hum Genet. 2020 Jan;65(2):175-180. doi: 10.1038/s10038-019-0686-1. PMID: 31636325. PMCID: PMC6920546.
- Yin W, Livraghi-Butrico A, Sears PR, Rogers TD, Burns KA, Grubb BR, Ostrowski LE. Mice with a Deletion of Rsph1 Exhibit a Low Level of Mucociliary Clearance and Develop a PCD Phenotype. Am J Respir Cell Mol Biol. 2019 Sep;61(3):312-321. doi: 10.1165/rcmb.2017-0387OC. PMID: 30896965. PMCID: PMC6839924.
- Bustamante-Marin XM, Yin WN, Sears PR, Werner ME, Brotslaw EJ, Mitchell BJ, Jania CM, Zeman KL, Rogers TD, Herring LE, Refabért L, Thomas L, Amselem S, Escudier E, Legendre M, Grubb BR, Knowles MR, Zariwala MA, Ostrowski LE. Lack of GAS2L2 Causes PCD by Impairing Cilia Orientation and Mucociliary Clearance. Am J Hum Genet. 2019 Feb 7;104(2):229-245. doi: 10.1016/j.ajhg.2018.12.009. PMID: 30665704. PMCID: PMC6372263.
- Rogers TD, Ostrowski LE, Livraghi-Butrico A, Button B, Grubb BR. Mucociliary Clearance in Mice Measured by Tracking Trans-tracheal Fluorescence of Nasally Aerosolized Beads. Sci Rep. 2018 Oct 3;8(1):14744. doi: 10.1038/s41598-018-33053-2. PMID: 30282981. PMCID: PMC6170422.
- Rosenfeld M, Ostrowski LE, Zariwala MA. Primary ciliary dyskinesia: Keep it on your radar. Thorax. 2018 Feb;73(2):101-102. doi: 10.1136/thoraxjnl-2017-210776. PMID: 29133352. PMCID: PMC6040643.
- Blackmon R, Kreda S, Sears P, Chapman B, Hill DB, Tracy J, Ostrowski L, Oldenburg A. Direct monitoring of pulmonary disease treatment biomarkers using plasmonic gold nanorods with diffusion-sensitive OCT. Nanoscale. 2017 Apr 13;9(15):4907-17. doi: 10.1039/c7nr00376e. PMID: 28358158. PMCID: PMC5473168.
- Blackburn K, Bustamante-Marin X, Yin W, Goshe MB, Ostrowski LE. Quantitative proteomic analysis of human airway cilia identifies previously uncharacterized proteins of high abundance. J Proteome Res. 2017 Apr 7;16(4):1579-1592. doi: 10.1021/acs.jproteome.6b00972. PMID: 28282151. PMCID: PMC5733142.
- Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4). doi: 10.1101/cshperspect.a028241. PMID: 27864314. PMCID: PMC5378048.
- Barrick J, Doblas A, Gardner MR, Sears PR, Ostrowski LE, Oldenburg AL. High-speed and high-sensitivity parallel spectral-domain optical coherence tomography using a supercontinuum light source. Opt Lett. 2016 Dec 15;41(24):5620-5623. doi: 10.1364/OL.41.005620. PMID: 27973473. PMCID: PMC5235345.
- Grubb BR, Livraghi-Butrico A, Rogers TD, Yin W, Button B, Ostrowski LE. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am J Physiol Lung Cell Mol Physiol. 2016 May 1;310(9):L860-7. doi: 10.1152/ajplung.00015.2016. PMID: 26968767. PMCID: PMC4867354.
- Turner MJ, Matthes E, Billet A, Ferguson AJ, Thomas DY, Randell SH, Ostrowski LE, Abbott-Banner K, Hanrahan JW. The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2016 Jan 1;310(1):L59-70. doi: 10.1152/ajplung.00324.2015. PMID: 26545902.
- Blackmon RL, Kreda SM, Sears PR, Ostrowski LE, Hill DB, Chapman BS, Tracy JB, Oldenburg AL. Diffusion-sensitive optical coherence tomography for real-time monitoring of mucus thinning treatments. Proc SPIE Int Soc Opt Eng. 2016;9697. PMID: 27746581. PMCID: PMC5061133.
- Sears PR, Yin WN, Ostrowski LE. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2015 Jul 15;309(2):L99-108. doi: 10.1152/ajplung.00024.2015. PMID: 25979076. PMCID: PMC4504973.
- Lin J, Yin W, Smith MC, Song K, Leigh MW, Zariwala MA, Knowles MR, Ostrowski LE, Nicastro D. Cryo-electron tomography reveals ciliary defects underlying human RSPH1 primary ciliary dyskinesia. Nat Commun. 2014 Dec 4;5:5727. doi: 10.1038/ncomms6727. PMID: 25473808; PMCID: PMC4267722.
- Knowles MR, Ostrowski LE, Leigh MW, Sears PR, Davis SD, Wolf WE, Hazucha MJ, Carson JL, Olivier KN, Sagel SD, Rosenfeld M, Ferkol TW, Dell SD, Milla CE, Randell SH, Yin W, Sannuti A, Metjian HM, Noone PG, Noone PJ, Olson CA, Patrone MV, Dang H, Lee HS, Hurd TW, Gee HY, Otto EA, Halbritter J, Kohl S, Kircher M, Krischer J, Bamshad MJ, Nickerson DA, Hildebrandt F, Shendure J, Zariwala MA. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med. 2014 Mar 15;189(6):707-17. doi: 10.1164/rccm.201311-2047OC. PMID: 24568568; PMCID: PMC3983840.
- Knowles MR, Ostrowski LE, Loges NT, Hurd T, Leigh MW, Huang L, Wolf WE, Carson JL, Hazucha MJ, Yin W, Davis SD, Dell SD, Ferkol TW, Sagel SD, Olivier KN, Jahnke C, Olbrich H, Werner C, Raidt J, Wallmeier J, Pennekamp P, Dougherty GW, Hjeij R, Gee HY, Otto EA, Halbritter J, Chaki M, Diaz KA, Braun DA, Porath JD, Schueler M, Baktai G, Griese M, Turner EH, Lewis AP, Bamshad MJ, Nickerson DA, Hildebrandt F, Shendure J, Omran H, Zariwala MA. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am J Hum Genet. 2013 Oct 3;93(4):711-20. doi: 10.1016/j.ajhg.2013.07.025. PMID: 24055112; PMCID: PMC3791252.
- Knowles MR, Leigh MW, Ostrowski LE, Huang L, Carson JL, Hazucha MJ, Yin W, Berg JS, Davis SD, Dell SD, Ferkol TW, Rosenfeld M, Sagel SD, Milla CE, Olivier KN, Turner EH, Lewis AP, Bamshad MJ, Nickerson DA, Shendure J, Zariwala MA; Genetic Disorders of Mucociliary Clearance Consortium. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013 Jan 10;92(1):99-106. doi: 10.1016/j.ajhg.2012.11.003. PMID: 23261302; PMCID: PMC3542458.
- Grubb BR, O’Neal WK, Ostrowski LE, Kreda SM, Button B, Boucher RC. Transgenic hCFTR expression fails to correct β-ENaC mouse lung disease. Am J Physiol Lung Cell Mol Physiol. 2012 Jan 15;302(2):L238-47. doi: 10.1152/ajplung.00083.2011. PMID: 22003093; PMCID: PMC3349361.
- Ostrowski LE, Yin W, Rogers TD, Busalacchi KB, Chua M, O’Neal WK, Grubb BR. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol. 2010 Jul;43(1):55-63. doi: 10.1165/rcmb.2009-0118OC. PMID: 19675306; PMCID: PMC2911571.
