Specialty Areas:
Animal models of inflammatory and obstructive lung diseases, lung mucosal immunology, mucin biology.
Chronology:
BS Cum Laude: University of Milan, 1996; PhD: University of Milan, 2001; Postdoctoral Research Associate: University of North Carolina at Chapel Hill, Cystic Fibrosis Research and Treatment Center, 2002- 2005; Research Associate: University of North Carolina at Chapel Hill, Cystic Fibrosis Research and Treatment Center, 2005-2013; Research Assistant Professor of Medicine: University of North Carolina at Chapel Hill, Marsico Lung Institute/Cystic Fibrosis Center, 2014-2020; Research Associate Professor of Medicine, University of North Carolina at Chapel Hill, Marsico Lung Institute/Cystic Fibrosis Center, 2021-present
Research Focus:
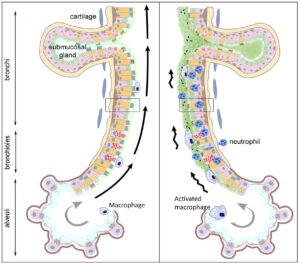
Our research focuses on understanding the molecular mechanisms involved in the derangement of mucus clearance and how its impairment impact other components of the airway mucosal immune system. Over the years, I have a developed a keen interest in using mouse models as a tool to understand the pathogenesis of obstructive lung disease, identify unique targets and test novel therapeutic approaches for translational research. In our lab, these studies nicely complement studies performed in available human specimens or simplified in vitro systems (e.g., primary cultures of airway epithelial cells differentiated at the air-liquid interface). Current research activities in the lab cover three main areas:
- Mouse models of defective airway mucus clearance are used to study responses to environmental and infectious challenges, to develop models of chronic airway infections, and to test efficiency and pharmacokinetic properties of mucokinetic/mucolytic/gene-editing therapies.
- Genetically engineered mouse models are used to study the contribution of specific components of the airway mucus clearance system (mucins secretion and assembly, ion, transport, cilia) in determining measurable phenotypes in both naïve and experimentally challenged conditions.
- Germ-free and specific pathogen-free mice are used to study the influence of age and host microbiota in the composition and function of airway mucus. These studies, funded by a recently awarded NHLBI R01, are complemented by efforts to define the pediatric airway epithelial landscape in healthy humans.
Affiliations (links):
Marsico Lung Institute/Cystic Fibrosis Center
Department of Microbiology and Immunology
Selected Bibliography:
- Joy N, Deshpande A, Lingamallu SM, Prabantu VM, Naveenkumar CN, Bharathkumar K, Bhat S, Alvarado-Martinez Z, Livraghi-Butrico A, Hagood JS, Boucher RC, Lafkas D, Byrd KM, Narayanan S, Shandil RK, Guha A. Notch signaling stabilizes lengths of motile cilia in multiciliated cells in the lung. Life Sci Alliance. 2026 Jan 22;9(3):e202503268. doi: 10.26508/lsa.202503268. PMID: 41571441; PMCID: PMC12827581.
- Schworer SA, Murano H, Dang H, Markovetz MR, Saito M, Kato T, Asakura T, Chen G, Gilmore RC, Morton LC, van Heusden C, Chua M, Strickler E, Wisniewski ZY, Crisp G, Mitchell E, Doherty KA, Mastan S, Trejo Bittar HE, Cody BA, Trudeau JB, De la Cruz G, Ralph LM, Askin FB, Panettieri RA Jr, Koziol-White CJ, Byrd KM, Livraghi-Butrico A, O’Neal WK, Randell SH, Wenzel SE, Okuda K, Boucher RC Jr. Airway Epithelial Heterogeneity and Mucus Plugging in Asthmatic Bronchioles. Am J Respir Crit Care Med. 2025 Sep 23. doi: 10.1164/rccm.202409-1849OC. Epub ahead of print. PMID: 40986379. PMCID: PMC12668797.
- Belfield-Simpson L, Martin JR, McPeek MK, Livraghi-Butrico A, Dang H, Kim YH, Gilmour MI, Doerschuk CM. Combustion products of burn pit constituents induce more changes in asthmatic than non-asthmatic murine lungs. Part Fibre Toxicol. 2025 Jul 29;22(1):21. doi: 10.1186/s12989-025-00625-w. PMID: 40734182; PMCID: PMC12305933.
- Edwards DA, Edwards A, Li D, Wang L, Chung KF, Bhatta D, Bilstein A, Hanes J, Endirisinghe I, Freeman BB, Gutay M, Livraghi-Butrico A, Button B. Global warming risks dehydrating and inflaming human airways. Commun Earth Environ. 2025;6(1):193. doi: 10.1038/s43247-025-02161-z. Epub 2025 Mar 17. PMID: 40678270; PMCID: PMC12269886.
- Heinzelmann K, Fysikopoulos A, Jaquin TJ, Peper-Gabriel JK, Hansbauer EM, Grüner S, Prassler J, Wurzenberger C, Kennedy JGC, Snead JY, Wrennall JA, Heinig K, Wurzenberger C, Bel Aiba RS, Tarran R, Livraghi-Butrico A, Fitzgerald MF, Anderson GP, Rothe C, Matschiner G, Olwill SA, Hagner M. Pulmonary-delivered Anticalin Jagged-1 antagonists reduce experimental airway mucus hyperproduction and obstruction. Am J Physiol Lung Cell Mol Physiol. 2025 Jan 1;328(1):L75-L92. doi: 10.1152/ajplung.00059.2024. PMID: 39499257. PMCID: PMC11905813.
- Nakano K, Whitehead GS, Lyons-Cohen MR, Grimm SA, Wilkinson CL, Izumi G, Livraghi-Butrico A, Cook DN, Nakano H. Chemokine CCL19 promotes type 2 T-cell differentiation and allergic airway inflammation. J Allergy Clin Immunol.2024 Feb;153(2):487-502.e9. doi: 10.1016/j.jaci.2023.10.024. PMID: 37956733. PMCID: PMC10922373.
- Donoghue LJ, Markovetz MR, Morrison CB, Chen G, McFadden KM, Sadritabrizi T, Gutay MI, Kato T, Rogers TD, Snead JY, Livraghi-Butrico A, Button B, Ehre C, Grubb BR, Hill DB, Kelada SNP. BPIFB1 loss alters airway mucus properties and diminishes mucociliary clearance. Am J Physiol Lung Cell Mol Physiol. 2023 Dec 1;325(6):L765-L775. doi: 10.1152/ajplung.00390.2022. PMID: 37847709. PMCID: PMC11068428.
- Yin W, Golliher HL, Ferguson AJ, Kimbell JS, Livraghi-Butrico A, Rogers TD, Grubb BR, Kimple AJ, Ostrowski LE. Mucolytic treatment of chronic rhinosinusitis in a murine model of primary ciliary dyskinesia. Front Mol Biosci. 2023 Jul 24;10:1221796. doi: 10.3389/fmolb.2023.1221796. PMID: 37555015; PMCID: PMC10405821.
- Mikami Y, Grubb BR, Rogers TD, Dang H, Asakura T, Kota P, Gilmore RC, Okuda K, Morton LC, Sun L, Chen G, Wykoff JA, Ehre C, Vilar J, van Heusden C, Livraghi-Butrico A, Gentzsch M, Button B, Stutts MJ, Randell SH, O’Neal WK, Boucher RC. Chronic airway epithelial hypoxia exacerbates injury in muco-obstructive lung disease through mucus hyperconcentration. Sci Transl Med. 2023 Jun 7;15(699):eabo7728. doi: 10.1126/scitranslmed.abo7728. PMID: 37285404. PMCID: PMC10664029.
- Livraghi-Butrico A, Franklin TB, Wolfgang MC. The rat takes the cheese: a novel model of CFTR-dependent chronic bacterial airway infection. Editorial. Eur Resp J. 2022 Sep 7;60(3):2200832. doi: 10.1183/13993003.00832-2022.
- Biering SB, Sarnik SA, Wang E, Zengel JR, Leist SR, Schäfer A, Sathyan V, Hawkins P, Okuda K, Tau C, Jangid AR, Duffy CV, Wei J, Gilmore RC, Alfajaro MM, Strine MS, Nguyenla X, Van Dis E, Catamura C, Yamashiro LH, Belk JA, Begeman A, Stark JC, Shon DJ, Fox DM, Ezzatpour S, Huang E, Olegario N, Rustagi A, Volmer AS, Livraghi-Butrico A, Wehri E, Behringer RR, Cheon DJ, Schaletzky J, Aguilar HC, Puschnik AS, Button B, Pinsky BA, Blish CA, Baric RS, O’Neal WK, Bertozzi CR, Wilen CB, Boucher RC, Carette JE, Stanley SA, Harris E, Konermann S, Hsu PD. Genome-wide bidirectional CRISPR screens identify mucins as host factors modulating SARS-CoV-2 infection. Nat Genet. 2022 Aug;54(8):1078-1089. doi: 10.1038/s41588-022-01131-x. PMID: 35879412; PMCID: PMC9355872.
- Kim N, Kwak G, Rodriguez J, Livraghi-Butrico A, Zuo X, Simon V, Han E, Shenoy SK, Pandey N, Mazur M, Birket SE, Kim A, Rowe SM, Boucher R, Hanes J, Suk JS. Inhaled gene therapy of preclinical muco-obstructive lung diseases by nanoparticles capable of breaching the airway mucus barrier. Thorax. 2022 Aug;77(8):812-820. doi: 10.1136/thoraxjnl-2020-215185. PMID: 34697091. PMCID: PMC9129924.
- Grubb BR, Livraghi-Butrico A. Animal models of cystic fibrosis in the era of highly effective modulator therapies. Review. Curr Opin Pharmacol. 2022 Jun;64:102235. doi: 10.1016/j.coph.2022.102235.PMID: 35576754; PMCID: PMC9386876.
- Rogers TD, Button B, Kelada SNP, Ostrowski LE, Livraghi-Butrico A, Gutay MI, Esther CR Jr, Grubb BR. Regional Differences in Mucociliary Clearance in the Upper and Lower Airways. Front Physiol. 2022 Mar 9;13:842592. doi: 10.3389/fphys.2022.842592. PMID: 35356083; PMCID: PMC8959816.
- Xu J, Livraghi-Butrico A, Hou X, Rajagopalan C, Zhang J, Song J, Jiang H, Wei HG, Wang H, Bouhamdan M, Ruan J, Yang D, Qiu Y, Xie Y, Barrett R, McClellan S, Mou H, Wu Q, Chen X, Rogers TD, Wilkinson KJ, Gilmore RC, Esther CR Jr, Zaman K, Liang X, Sobolic M, Hazlett L, Zhang K, Frizzell RA, Gentzsch M, O’Neal WK, Grubb BR, Chen YE, Boucher RC, Sun F. Phenotypes of CF rabbits generated by CRISPR/Cas9-mediated disruption of the CFTR gene. JCI Insight. 2021 Jan 11;6(1):e139813. doi: 10.1172/jci.insight.139813. PMID: 33232302. PMCID: PMC7821608.
- Ehre C, Rushton ZL, Wang B, Hothem LN, Morrison CB, Fontana NC, Markovetz MR, Delion MF, Kato T, Villalon D, Thelin WR, Esther CR Jr, Hill DB, Grubb BR, Livraghi-Butrico A, Donaldson SH, Boucher RC. An Improved Inhaled Mucolytic to Treat Airway Muco-Obstructive Diseases. Am J Respir Crit Care Med. 2019 Jan 15;199(2):171-180. doi: 10.1164/rccm.201802-0245OC. PMID: 30212240. PMCID: PMC6353008.
- Chen G, Volmer AS, Wilkinson KJ, Deng Y, Jones LC, Yu D, Bustamante-Marin XM, Burns KA, Grubb BR, O’Neal WK, Livraghi-Butrico A, Boucher RC. Role of Spdef in the regulation of Muc5b expression in the airways of naïve and muco-obstructed mice. Am J Respir Cell Mol Biol. 2018 Sep;59(3):383-396. PMID: 29579396. PMCID: PMC6189647.
- Livraghi-Butrico A, Wilkinson KJ, Volmer AS, Gilmore RC, Rogers TD, Caldwell RA, Burns KA, Esther CR Jr, Mall MA, Boucher RC, O’Neal WK, Grubb BR. Lung disease phenotypes caused by over-expression of combinations of alpha, beta, and gamma subunits of the epithelial sodium channel in mouse airways. Am J Physiol Lung Cell Mol Physiol 2018 Feb 1;314(2):L318-L331. PMID: 29074490. PMCID: PMC5866504.
- Donoghue LJ, Livraghi-Butrico A, McFadden KM, Thomas JM, Chen G, Grubb BR, O’Neal WK, Boucher RC, Kelada SNP. Identification of trans protein QTL for secreted airway mucins in mice and a causal role for Bpifb1. Genetics 2017 Oct;207(2):801-812. PMID: 28851744. PMCID: PMC5629340.
- Livraghi-Butrico A, Grubb BR, Wilkinson KJ, Volmer AS, Burns KA, Evans CM, O’Neal WK, Boucher RC. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol 2017;10(2):395-407. PMID: 27435107. PMCID: PMC5250616.
- Grubb BR, Livraghi-Butrico A, Rogers TD, Yin W, Button B, Ostrowski LE. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5B mucin. Am J Physiol Lung Physiol 2016 May 1;310(9):L860-7. PMID: 26968767. PMCID: PMC4867354.
- Roy MG*, Livraghi-Butrico A*, Fletcher AA*, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defense. Nature 2014 Jan 16;505(7483):412-6. PMID: 24317696. PMCID: PMC4001806.
- Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O’Neal WK, Grubb BR. Loss of Cftr function exacerbates the phenotype of Na+ hyperabsorption in murine airways. Am J Physiol Lung Cell Mol Physiol 2013 Apr 1;304(7):L469-80. PMID: 23377346. PMCID: PMC3627939.
- Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O’Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol 2012 Jul 5(4):397-408. PMID: 22419116. PMCID: PMC3377774.
- Livraghi-Butrico A, Grubb BR, Kelly EJ, Wilkinson KJ, Yang H, Geiser M, Randell SH, Boucher RC, O’Neal WK. Genetically determined heterogeneity of lung disease in a mouse model of airway mucus obstruction. Physiol Genomics 2012 Apr 15;44(8):470-84. PMID: 22395316. PMCID: PMC3339860.
- Livraghi A, Mall M, Paradiso AM, Boucher RC, Ribeiro CM. Modeling dysregulated Na+ absorption in airway epithelial cells with mucosal nystatin treatment. Am J Respir Cell Mol Biol 2008 Apr;38(4):423-34. PMID: 17989361. PMCID: PMC2274946.
Complete List of Published Work in MyBibliography:
