Specialty Areas:
Genetic disorders of mucociliary clearance, including gene modifiers of disease phenotype in cystic fibrosis (CF) lung and liver disease, primary ciliary dyskinesia (PCD), as well as idiopathic bronchiectasis.
Chronology:
AB: University of North Carolina, 1967; MD: University of North Carolina, 1971; Resident: Ohio State University, 1971-1973; Resident and Chief Resident: Duke University Medical Center and Veteran’s Administration Hospital, 1973-1975; Chief Internal Medicine: Malcolm Grow Medical Center, 1975-1978; Fellow: University of North Carolina, 1978-1980; Instructor: University of North Carolina, 1980-1982; Assistant Professor: University of North Carolina, 1982-1987; Associate Professor: University of North Carolina, 1987-1994; Professor of Medicine: University of North Carolina, 1994-present.
Research Focus:
Dr. Michael R. Knowles is a Professor of Pulmonary and Critical Care Medicine at UNC. He has over 3 decades of clinical research experience that spans across the disciplines of biology, physiology, genetics and clinical trials in both the academic and private sectors. He is currently the head of two large multicenter studies: 1) Genetic Modifiers of Disease phenotype (severity) in cystic fibrosis lung and liver disease, which includes an International Consortium doing whole genome and differential gene expression studies; and 2) a Consortium with 8 sites in North America to study rare genetic disorders of mucociliary clearance, and non-CF/non-PCD (idiopathic) bronchiectasis.
Links:
UNC PCD Research and Treatment
Genetic Diseases of Mucociliary Clearance Consortium
Rare Clinical Diseases Research Network
Genetic Disorders of Mucociliary Clearance – NIH/NHLBI (Knowles).
The major goal of this project is to establish a network of geographically-dispersed clinical research sites that are designed to study rare diseases of the airways. These sites will collaborate in diagnostic, genetic, and a range of other studies in mucociliary clearance, including primary ciliary dyskinesia (PCD). A major focus is the identification of disease-causing genes for PCD. This Consortium is also studying idiopathic bronchiectasis in patients with, and without, non-tuberculous myobacteria (NTM).
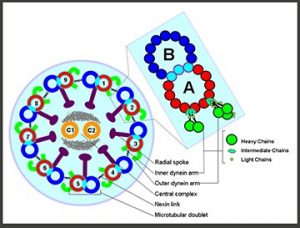
Fig 1. Schematic drawing of the eukaryotic cilium.
Genetic Modifiers in CF Lung Disease. NIH/NHLBI (Knowles, O’Neal, Dang).
The major goals of this study are to identify associations between non-CFTR genes and the pulmonary phenotype, and test for the association of candidate modifier alleles with severity (or mildness) of pulmonary disease. Identification of genes that modulate the severity of the pulmonary phenotype will improve understanding of the pathophysiology of CF lung disease and identify new targets for therapeutic intervention.
Genetic Modifiers in CF Liver Disease (Knowles and Stonebraker).
This study examines “modifier genes” that may play a role in the development of CF liver disease. Modifiers are genes, other than the CF gene (CFTR), which may directly or indirectly have an effect on how the body responds to the conditions that develop as the result of the defective CFTR gene. The identification of modifier genes that influence disease severity may ultimately lead to a better understanding of CF liver disease, and may be useful in the development of new treatments.
Pathogenesis of PCD Lung Disease. NIH/NHLBI (Knowles, Leigh, Ostrowski, Zariwala).
The major goals of this project are to define the ciliary physiologic phenotype of PCD, and investigate the molecular, pathogenesis of lung disease associated with defective MCC, including underlying genetic causes of disease.
Molecular Phenotypes for Cystic Fibrosis Lung Disease. NIH/NHLBI (Knowles, Wright, Zou, Dang, O’Neal).
The major goal of this project is to define a robust molecular phenotype for CF lung disease, which relates to prognosis, and new targets for therapy.
Bronchiectasis Research Registry. COPD Foundation, (Knowles, Daniels, Noone).
This project is to fund creation and maintenance of a database of patient information for bronchiectasis research.
Collaborations: Collaborations are essential to the research currently undertaken in the Knowles research lab. These include investigators and patients from both national and international sites. The world-wide CF and PCD communities have come together to aid in the projects below. While we are unable to list all the sites who have contributed, the founding members include the following investigators.
Genetic Modifiers of CF Liver and Lung Disease Research: International CF Modifier Consortium (coordinating members include):
The University of North Carolina at Chapel Hill, Mike Knowles, MD
Case Western Reserve University, Cleveland, OH, Mitch Drumm, PhD
Johns Hopkins University, Baltimore, MD, Garry Cutting, MD
The Hospital for Sick Children, Toronto, Canada, Lisa Strug, PhD, Simon Ling, MD
Trousseau Hospital, Paris, France, Harriet Corvol, MD
Primary Ciliary Dyskinesia Research: Genetic Disorders of Mucociliary Clearance Consortium (coordinating members include):
University of South Florida, DMCC, Jeff Krischer, PhD
Washington University in St. Louis, Tom Ferkol, MD, Jeff Atkinson, MD, and Steve Brody, MD
The Children’s Hospital Colorado, Aurora, Scott Sagel, MD
National Jewish Health, Denver, Charles Daley, MD
Stanford University, Carlos Milla, MD
Children’s Hospital & Regional Medical Center, Seattle, Margaret Rosenfeld, MD
The Hospital for Sick Children, Toronto, Canada, Sharon Dell, MD
NIH NHLBI, Ken Olivier, MD
Indiana University, Stephanie Davis, MD
McGill University, Montreal, Adam Shapiro, MD
Cecilia Lo, PhD, University of Pittsburg
International
Heymut Omran, MD, Munster, Germany
Jane Lucas, MD, South Hampton U. Hospital NHS Trust, UK
Lucy Morgan, University of Sydney, Australia
Estelle Escudier, MD, Unite Inserm, Faculte de Medecine de Creteil, Cedex, France
Serge Amselem, MD, Unite Inserm, Faculte de Medecine de Creteil, Cedex, France
Selected Bibliography:
- Leigh MW, Shapiro AJ, Chawla KK, Hazucha MJ, Brown DE, Lin FC, Jiang L, Carson JL, Davis SD, Dell SD, Sagel SD, Rosenfeld M, Milla C, Sullivan KM, Zariwala MA, Ferkol TW, Knowles MR; Genetic Disorders of Mucociliary Clearance Consortium. Tidal Breathing Nasal Nitric Oxide Measurement as a Test for Primary Ciliary Dyskinesia in Young Children. Eur Respir J. 2026 Jan 29:2501540. doi: 10.1183/13993003.01540-2025. Epub ahead of print. PMID: 41611251.
- Ostrowski LE, Abu-Nasser S, Zeman KL, Leigh MW, Zariwala MA, Olivier KN, Ferkol TW, Taylor CN, Ceppe AS, Knowles MR, Bennett WD. Mucociliary and cough clearance in primary ciliary dyskinesia as affected by mutations in RSPH1 or DNAH5. ERJ Open. 2026 Jan 19;12(1):00681-2025. doi: 10.1183/23120541.00681-2025. PMCID: PMC12813685.
- Bourassa MH, Sillon G, Ding S, Chioccioli M, Lek M, Ma K, Mejia-Garcia A, Gravel S, Vinh DC, Knowles MR, Leigh MW, Davis SD, Ferkol T, Olivier KN, Schecterman EN, Yin W, Sears PR, Gentzsch M, Boyles SE, Bennett WD, Zeman KL, Ostrowski LE, Zariwala MA, Shapiro AJ. ODAD4-related primary ciliary dyskinesia: Report of five cases and a founder variant in Quebec. Cells. 2025 Sep 18;14(18):1460. doi: 10.3390/cells14181460. PMID: 41002425; PMCID: PMC12468610.
- Kinghorn B, Mollica F, Caudri D, Davis SD, Dell S, Ferkol TW, Gardner RA, Knowles MR, Milla C, Pittman JE, Rosenfeld M, Sagel SD, Shapiro AJ, Sullivan E, Sullivan KM, Tiddens HAWM, Zariwala MA, Leigh MW; Genetic Disorders of Mucociliary Clearance Consortium. Airway disease progression on chest computed tomography in children with primary ciliary dyskinesia. Pediatr Pulmonol. 2025 Sep;60(9):e71287. doi: 10.1002/ppul.71287. PMID: 40965845.
- Barber AT, Davis SD, Ferkol TW, Shapiro AJ, Atkinson J, Sagel SD, Dell SD, Olivier K, Milla C, Rosenfeld M, Li L, Lin FC, Sullivan KM, Capps NA, Zariwala MA, Knowles MR, Leigh MW; Genetic Disorders of Mucociliary Clearance Consortium (GDMCC). The association of neonatal respiratory distress with ciliary ultrastructure and genotype in primary ciliary dyskinesia. Pediatr Pulmonol. 2025 May;60(5):e71091. doi: 10.1002/ppul.71091. PMID: 40344341; PMCID: PMC12063519.
- Faino AV, Gordon WW, Buckingham K, Stilp AM, Pace RG, Raraigh KS, Collaco JM, Zhou YH, Dang H, O’Neal W, Knowles MK, Cutting GR, Rosenfeld M, Bamshad MJ, Gibson RL, Blue EE; Cystic Fibrosis Genome Project. CHP2 modifies chronic Pseudomonas aeruginosa airway infection risk in cystic fibrosis. Ann Am Thorac Soc. 2025 May;22(5):715-723. doi: 10.1513/AnnalsATS.202408-868OC.PMID: 39746161. PMCID: PMC12392791.
- Beaman MM, Yin W, Smith AJ, Sears PR, Leigh MW, Ferkol TW, Kearney B, Olivier KN, Kimple AJ, Clarke S, Huggins E, Nading E, Jung SH, Iyengar AK, Zou X, Dang H, Barrera A, Majoros WH, Rehder CW, Reddy TE, Ostrowski LE, Allen AS, Knowles MR, Zariwala MA, Crawford GE. Promoter deletion leading to allele specific expression in a genetically unsolved case of primary ciliary dyskinesia. Am J Med Genet A. 2025 Feb;197(2):e63880. doi: 10.1002/ajmg.a.63880. PMID: 39364610. PMCID: PMC11698635.
- Farzal Z, Sullivan KM, Zariwala MA, Thorp BD, Senior BA, Ebert CS Jr, Davis S, Leigh MW, Knowles MR, Kimple AJ. Olfactory Dysfunction in Primary Ciliary Dyskinesia. OTO Open. 2025 Jan 31;9(1):e70084. doi: 10.1002/oto2.70084. PMID: 39896853; PMCID: PMC11783683.
- Stonebraker JR, Pace RG, Gallins PJ, Dang H, Aksit MA, Faino AV, Gordon WW, MacParland S, Bamshad MJ, Gibson RL, Cutting GR, Durie PR, Wright FA, Zhou YH, Blackman SM, O’Neal WK, Ling SC, Knowles MR. Genetic variation in severe cystic fibrosis liver disease is associated with novel mechanisms for disease pathogenesis. Hepatology. 2024 Nov; 80(5):1012-1025. doi: 10.1097/HEP.0000000000000863. PMID: 38536042. PMCID: PMC11427593.
- Dougherty GW, Ostrowski LE, Nöthe-Menchen T, Raidt J, Schramm A, Olbrich H, Yin W, Sears PR, Dang H, Smith AJ, Beule AG, Hjeij R, Rutjies N, Haarman EG, Maas SM, Ferkol TW, Noone PG, Olivier KN, Bracht DC, Barbry P, Zaragosi LE, Fierville M, Kliesch S, Wohlgemuth K, König J, George S, Loges NT, Ceppe A, Markovetz MR, Luo H, Guo T, Rizk H, Eldesoky T, Dahlke K, Boldt K, Ueffing M, Hill DB, Pang YP, Knowles MR, Zariwala MA, Omran H. Recessively inherited deficiency of secreted WFDC2 (HE4) causes nasal polyposis and bronchiectasis. Am J Respir Crit Care Med. 2024 Jul 1;210(1):63-76. doi: 10.1164/rccm.202308-1370OC. PMID: 38626355. PMCID: PMC11197063.
- Dodd DO, Mechaussier S, Yeyati PL, McPhie F, Anderson JR, Khoo CJ, Shoemark A, Gupta DK, Attard T, Zariwala MA, Legendre M, Bracht D, Wallmeier J, Gui M, Fassad MR, Parry DA, Tennant PA, Meynert A, Wheway G, Fares-Taie L, Black HA, Mitri-Frangieh R, Faucon C, Kaplan J, Patel M, McKie L, Megaw R, Gatsogiannis C, Mohamed MA, Aitken S, Gautier P, Reinholt FR, Hirst RA, O’Callaghan C, Heimdal K, Bottier M, Escudier E, Crowley S, Descartes M, Jabs EW, Kenia P, Amiel J, Bacci GM, Calogero C, Palazzo V, Tiberi L, Blümlein U, Rogers A, Wambach JA, Wegner DJ, Fulton AB, Kenna M, Rosenfeld M, Holm IA, Quigley A, Hall EA, Murphy LC, Cassidy DM, von Kriegsheim A; Scottish Genomes Partnership16; Genomics England Research Consortium45; Undiagnosed Diseases Network46; Papon JF, Pasquier L, Murris MS, Chalmers JD, Hogg C, Macleod KA, Urquhart DS, Unger S, Aitman TJ, Amselem S, Leigh MW, Knowles MR, Omran H, Mitchison HM, Brown A, Marsh JA, Welburn JPI, Ti SC, Horani A, Rozet JM, Perrault I, Mill P. Ciliopathy patient variants reveal organelle-specific functions for TUBB4B in axonemal microtubules. Science. 2024 Apr 26;384(6694):eadf5489. doi: 10.1126/science.adf5489. PMID: 38662826. PMCID: PMC7616230.
- Ringshausen FC, Shapiro AJ, Nielsen KG, Mazurek H, Pifferi M, Donn KH, van der Eerden MM, Loebinger MR, Zariwala MA, Leigh MW, Knowles MR, Ferkol TW; CLEAN-PCD investigators and study team. Safety and efficacy of the epithelial sodium channel blocker idrevloride in people with primary ciliary dyskinesia (CLEAN-PCD): A multinational, phase 2, randomised, double-blind, placebo-controlled crossover trial. Lancet Respir Med. 2024 Jan;12(1):21-33. doi: 10.1016/S2213-2600(23)00226-6. PMID: 37660715.
- Zhou YH, Gallins PJ, Pace RG, Dang H, Aksit MA, Blue EE, Buckingham KJ, Collaco JM, Faino AV, Gordon WW, Hetrick KN, Ling H, Liu W, Onchiri FM, Pagel K, Pugh EW, Raraigh KS, Rosenfeld M, Sun Q, Wen J, Li Y, Corvol H, Strug LJ, Bamshad MJ, Blackman SM, Cutting GR, Gibson RL, O’Neal WK, Wright FA, Knowles MR. Genetic Modifiers of Cystic Fibrosis Lung Disease Severity: Whole Genome Analysis of 7,840 Patients. Am J Respir Crit Care Med. 2023 May 15;207(10):1324-1333. doi: 10.1164/rccm.202209-1653OC. PMID: 36921087. PMCID: PMC10595435.
- Sagel SD, Kupfer O, Wagner BD, Davis SD, Dell SD, Ferkol TW, Hoppe JE, Rosenfeld M, Sullivan KM, Tiddens HAWM, Knowles MR, Leigh MW; Genetic Disorders of Mucociliary Clearance Consortium. Airway Inflammation in Children with Primary Ciliary Dyskinesia. Ann Am Thorac Soc. 2023 Jan;20(1):67-74. doi: 10.1513/AnnalsATS.202204-314OC. PMID: 35984413. PMCID: PMC9819265.
- Shapiro AJ, Sillon G, D’Agostino D, Baret L, López-Giráldez F, Mane S, Leigh MW, Davis SD, Knowles MR, Zariwala MA. HYDIN variants are a common cause of primary ciliary dyskinesia in French-Canadians. Ann Am Thorac Soc. 2023 Jan;20(1):140-144. doi: 10.1513/AnnalsATS.202203-253RL. PMID: 36112114. PMCID: PMC9819264.
- Wee WB, Leigh MW, Davis SD, Rosenfeld M, Sullivan KM, Sawras MG, Ferkol TW, Knowles MR, Milla C, Sagel SD, Zariwala MA, Pullenayegum E, Dell SD. Association of neonatal hospital length of stay with lung function in primary ciliary dyskinesia. Ann Am Thorac Soc. 2022 Nov;19(11):1865-1870. doi: 10.1513/AnnalsATS.202202-116OC. PMID: 35657736. PMCID: PMC9667809.
- Fecho K, Ahalt SC, Knowles M, Krishnamurthy A, Leigh M, Morton K, Pfaff E, Wang M, Yi H. Leveraging open electronic health record data and environmental exposures data to derive insights into rare pulmonary disease. Front Artif Intell. 2022 Jun 28;5:918888. doi: 10.3389/frai.2022.918888. PMID: 35837616; PMCID: PMC9274244.
- Raraigh KS, Aksit MA, Hetrick K, Pace RG, Ling H, O’Neal W, Blue E, Zhou YH, Bamshad MJ, Blackman SM, Gibson RL, Knowles MR, Cutting GR. Complete CFTR gene sequencing in 5,058 individuals with cystic fibrosis informs variant-specific treatment. J Cyst Fibros. 2022 May;21(3):463-470. doi: 10.1016/j.jcf.2021.10.011. PMID: 34782259.
- Ostrowski LE, Yin W, Smith AJ, Sears PR, Bustamante-Marin XM, Dang H, Hildebrandt F, Daniels LA, Capps NA, Sullivan KM, Leigh MW, Zariwala MA, Knowles MR. Expression of a truncated form of ODAD1 associated with an unusually mild primary ciliary dyskinesia phenotype. Int J Mol Sci. 2022 Feb 3;23(3):1753. doi: 10.3390/ijms23031753. PMID: 35163670. PMCID: PMC8835943.
- Eastman AC, Pace RG, Dang H, Aksit MA, Vecchio-Pagán B, Lam AN, O’Neal WK, Blackman SM, Knowles MR*, Cutting GR*. SLC26A9 SNP rs7512462 is not associated with lung disease severity or lung function response to ivacaftor in cystic fibrosis patients with G551D-CFTR. J Cyst Fibros. 2021 Sep;20(5):851-856. doi: 10.1016/j.jcf.2021.02.007.PMID: 33674211. PMCID: PMC8410892. *Co-senior authors.
- Pappa AK, Sullivan KM, Lopez EM, Adams KN, Zanation AM, Ebert CS Jr, Thorp BD, Senior BA, Leigh MW, Knowles MR, Kimple AJ. Sinus development and pneumatization in a primary ciliary dyskinesia cohort. Am J Rhinol Allergy. 2021 Jan;35(1):72-76. doi: 10.1177/1945892420933175. PMID: 32551925. PMCID: PMC7750665.
- Dang H, Polineni D, Pace RG, Stonebraker JR, Corvol H, Cutting GR, Drumm ML, Strug LJ, O’Neal WK, Knowles MR. Mining GWAS and eQTL data for CF lung disease modifiers by gene expression imputation. PLoS One. 2020 Nov 30;15(11):e0239189. doi: 10.1371/journal.pone.0239189. PMID: 33253230.. PMCID: PMC7703903.
- Aksit MA, Pace RG, Vecchio-Pagan B, Ling H, Rommens JM, Boelle P, L Guillot, Raraigh KS, E Pugh, Zhang P, Strug LJ, Drumm ML, Knowles MR, Cutting GR, Corvol H, Blackman SM. Genetic modifiers of cystic fibrosis-related diabetes have extensive overlap with type 2 diabetes and related traits. J Clin Endocrinol Metab. 2020 May 1;105(5). doi: 10.1210/clinem/dgz102. PMID: 31697830. PMCID: PMC7236628.
- Chivukula RR, Montoro DT, Leung HM, Yang J, Shamseldin HE, Taylor MS, Dougherty GW, Zariwala MA, Carson J, Daniels MLA, Sears PR, Black KE, Hariri LP, Almogarri I, Frenkel EM, Vinarsky V, Omran H, Knowles MR, Tearney GJ, Alkuraya FS, Sabatini DM. A human ciliopathy reveals essential functions for NEK10 in airway mucociliary clearance. Nat Med. 2020 Feb;26(2):244-251. doi: 10.1038/s41591-019-0730-x. PMID: 31959991. PMCID: PMC7018620.
- Bustamante-Marin XM, Shapiro A, Sears PR, Charng WL, Conrad DF, Leigh MW, Knowles MR, Ostrowski LE, Zariwala MA. Identification of genetic variants in CFAP221 as a cause of primary ciliary dyskinesia. J Hum Genet. 2020 Jan;65(2):175-180. doi: 10.1038/s10038-019-0686-1. PMID: 31636325. PMCID: PMC6920546.
- Shapiro AJ, Ferkol TW, Manion M, Leigh MW, Davis SD, Knowles MR. High-Speed Videomicrosopy Analysis Presents Limitations in Diagnosis of Primary Ciliary Dyskinesia. Am J Respir Crit Care Med. 2020 Jan 1;201(1):122-123. doi: 10.1164/rccm.201907-1366LE. PMID: 31433949. PMCID: PMC6938157.
- Vece TJ, Sagel SD, Zariwala MA, Sullivan KM, Burns KA, Dutcher SK, Yusupov R, Leigh MW, Knowles MR. Cytoplasmic “ciliary inclusions” in isolation are not sufficient for the diagnosis of primary ciliary dyskinesia. Pediatr Pulmonol. 2020 Jan;55(1):130-135. doi: 10.1002/ppul.24528.PMID: 31549486. PMCID: PMC7068840.
- Wallmeier J, Frank D, Shoemark A, Nöthe-Menchen T, Cindric S, Olbrich H, Loges NT, Aprea I, Dougherty GW, Pennekamp P, Kaiser T, Mitchison HM, Hogg C, Carr SB, Zariwala MA, Ferkol T, Leigh MW, Davis SD, Atkinson J, Dutcher SK, Knowles MR, Thiele H, Altmüller J, Krenz H, Wöste M, Brentrup A, Ahrens F, Vogelberg C, Morris-Rosendahl DJ, Omran H. De novo mutations in FOXJ1 result in a motile ciliopathy with hydrocephalus and randomization of left/right body asymmetry. Am J Hum Genet. 2019 Nov 7;105(5):1030-1039. doi: 10.1016/j.ajhg.2019.09.022. PMID: 31630787. PMCID: PMC6849114.
- Shapiro AJ, Leigh MW, Omran H, Lavergne V, Knowles MR. Errors in methodology affect diagnostic accuracy of high-speed videomicroscopy analysis in primary ciliary dyskinesia. Chest. 2019 Nov;156(5):1032-1033. doi: 10.1016/j.chest.2019.06.021. PMID: 31699224. PMCID: PMC7339238.
- Hannah WB, DeBrosse S, Kinghorn B, Strausbaugh S, Aitken ML, Rosenfeld M, Wolf WE, Knowles MR, Zariwala MA. The expanding phenotype of OFD1-related disorders: Hemizygous loss-of-function variants in three patients with primary ciliary dyskinesia. Mol Genet Genomic Med. 2019 Sep;7(9):e911. doi: 10.1002/mgg3.911. PMID: 31373179. PMCID: PMC6732318.
- Morimoto K, Hijikata M, Zariwala M, Nykamp K, Inaba A, Guo TC, Yamada,H, Truty R, Sasaki Y, Ohta K, Kudoh S, Leigh M, Knowles MR, Keicho N. Recurring large deletion in DRC1 (CCDC164) identified as causing primary ciliary dyskinesia in two Asian patients. Mol Genet Genom Med. 2019 Aug;7(8):e838. doi: 10.1002/mgg3.838. PMID: 31270959. PMCID: PMC6687623.
- Gong J, Wang F, Xiao B, Panjwan Ni, Lin F, Keenan K, Avolio J, Esmaeili M, Zhang L, He G, Soave D, Mastromatteo S, Baskurt Z, Kim S, O’Neal WK, Polineni D, Blackman SM, Corvol H, Cutting GR, Drumm M, Knowles MR, Rommens JM, Sun L, Strug LJ. Genetic association and transcriptome integration identify contributing genes and tissues at cystic fibrosis modifier loci. PLoS Genet. 2019 Feb 26;15(2):e1008007. doi: 10.1371/journal.pgen.1008007. PMID: 30807572. PMCID: PMC6407791.
- Bustamante-Marin XM, Yin WN, Sears PR, Werner ME, Brotslaw EJ, Mitchell BJ, Jania CM, Zeman KL, Rogers TD, Herring LE, Refabért L, Thomas L, Amselem S, Escudier E, Legendre M, Grubb BR, Knowles MR, Zariwala MA, Ostrowski LE. Lack of GAS2L2 Causes PCD by Impairing Cilia Orientation and Mucociliary Clearance. Am J Hum Genet. 2019 Feb 7;104(2):229-245. doi: 10.1016/j.ajhg.2018.12.009. PMID: 30665704. PMCID: PMC6372263.
- Davis SD, Rosenfeld M, Lee HS, Ferkol TW, Sagel SD, Dell SD, Milla C, Pittman JE, Shapiro AJ, Sullivan KM, Nykamp KR, Krischer JP, Zariwala MA, Knowles MR, Leigh MW. Primary Ciliary Dyskinesia: Longitudinal Study of Lung Disease by Ultrastructure Defect and Genotype. Am J Respir Crit Care Med. 2019 Jan 15;199(2):190-198. doi: 10.1164/rccm.201803-0548OC. PMID: 30067075. PMCID: PMC6353004.
- Polineni D, Piccorelli AV, Hannah WB, Dalrymple SN, Pace RG, Durie PR, Ling SC, Knowles MR, Stonebraker JR. Analysis of a large cohort of cystic fibrosis patients with severe liver disease indicates lung function decline does not significantly differ from that of the general cystic fibrosis population. PLoS One. 2018 Oct 11;13(10):e0205257. doi: 10.1371/journal.pone.0205257. PMID: 30307979. PMCID: PMC6181334.
- O’Neal WK, Knowles MR. Cystic fibrosis disease modifiers: Complex genetics defines the phenotypic diversity in a monogenic disease. Annu Rev Genomics Hum Genet. 2018 Aug 31;19:201-222. doi: 10.1146/annurev-genom-083117-021329. PMID: 29709203.
- Knowles MR, Leigh MW. Primary Ciliary Dyskinesia Diagnosis. Is Color Better Than Black and White? Am J Respir Crit Care Med. 2017 Jul 1;196(1):9-10. doi: 10.1164/rccm.201702-0426ED. PMID: 28665204.
- Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, Dell SD, Chilvers MA, Ferkol TW, Zariwala MA, Sagel SD, Josephson M, Morgan L, Yilmaz O, Olivier KN, Milla C, Pittman JE, Daniels LA, Jones MH, Janahi IA, Ware SM, Daniel SJ, Cooper ML, Nogee LM, Anton B, Eastvold T, Ehrne L, Guadagno E, Knowles MR, Leigh MW, Lavergne V; on behalf of the ATS Assembly on Pediatrics. Diagnosis of primary ciliary dyskinesia: An official ATS clinical practice guideline. Am J Respir Crit Care Med. 2018 Jun 15;197(12):e24-e39. doi: 10.1164/rccm.201805-0819ST. PMID: 29905515. PMCID: PMC6006411.
- Leigh MW, Knowles MR. Assessment of Ciliary Beat Pattern: Variability in Healthy Control Subjects Has Implications for Use as Test for Primary Ciliary Dyskinesia. Chest. 2017 May;151(5):958-959. doi: 10.1016/j.chest.2016.11.025. PMID: 28483130.
- Panjwani N, Xiao B, Xu L, Gong J, Keenan K, Lin F, He G, Baskurt Z, Kim S, Zhang L, Esmaeili M, Blackman S, Paterson A, Scherer S, Corvol H, Drumm M, Knowles M, Cutting G, Rommens JM, Sun L, Strug LJ. Improving imputation in disease-relevant regions: Lessons from cystic fibrosis. NPJ Genom Med. 2018 Mar 20;3:8. doi: 10.1038/s41525-018-0047-6. PMID: 29581887. PMCID: PMC5861096.
- Polineni D, Dang H, Jones L, Gallins P, Pace R, Stonebraker J, Commander L, Krenicky J, Zhou Y, Corvol H, Cutting G, Drumm M, Strug L, Boyle M, Durie P, Chmiel J, Zou F, Wright F, O’Neal W, Knowles M. Airway mucosal host defense is key to genomic regulation of cystic fibrosis lung disease severity. Am J Respir Crit Care Med. 2018 Jan 1;197(1):79-93. doi: 10.1164/rccm.201701-0134OC. PMID: 28853905. PMCID: PMC5765386.
- Pittman JE, Noah H, Calloway HE, Davis SD, Leigh MW, Drumm M, Sagel SD, Accurson FJ, Knowles MR, Sontag MK. Early childhood lung function is a stronger predictor than early Pseudomonas aeruginosa infection of adolescent lung function in cystic fibrosis. PLoS One. 2017 May 15;12(5):e0177215. doi: 10.1371/journal.pone.0177215. eCollection 2017. PMID: 28505188. PMCID: PMC5432103.
- Daniels ML, Birchard KR, Lowe JR, Patrone MV, Noone PG, Knowles MR. Enlarged dural sac in idiopathic bronchiectasis implicates heritable connective tissue gene variants. Ann Am Thorac Soc. 2016 Oct;13(10):1712-1720. PMID: 27409985; PMCID: PMC5122488.
- Dell SD, Leigh MW, Lucas JS, Ferkol TW, Knowles MR, Alpern A, Behan L, Morris AM, Hogg C, DunnGalvin A, Quittner AL. Primary ciliary dyskinesia: First health-related quality-of-life measures for pediatric patients. Ann Am Thorac Soc. 2016 Oct;13(10):1726-1735. PMID: 27464304; PMCID: PMC5122491.
- Knowles MR, Zariwala M, Leigh M. Primary ciliary dyskinesia. Clin Chest Med. 2016 Sep;37(3):449-461. doi: 10.1016/j.ccm.2016.04.008. Review. PMID: 27514592; PMCID: PMC4988337.
- Leigh MW, Ferkol TW, Davis SD, Lee HS, Rosenfeld M, Dell SD, Sagel SD, Milla C, Olivier KN, Sullivan KM, Zariwala MA, Pittman JE, Shapiro AJ, Carson JL, Krischer J, Hazucha MJ, Knowles MR. Clinical features and associated likelihood of primary ciliary dyskinesia in children and adolescents. Ann Am Thorac Soc. 2016 Aug;13(8):1305-1313. doi: 10.1513/AnnalsATS.201511-748OC. PMID: 27070726; PMCID: PMC5021075.
- Stonebraker JR, Ooi CY, Pace RG, Corvol H, Knowles MR, Durie PR, Ling SC. Features of severe liver disease with portal hypertension in patients with cystic fibrosis. Clin Gastroenterol Hepatol. 2016 Aug;14(8):1207-1215.e3. doi: 10.1016/j.cgh.2016.03.041. PMID: 27062904; PMCID: PMC4955685.
- Shapiro AJ, Zariwala MA, Ferkol T, Davis SD, Sagel SD, Dell SD, Rosenfeld M, Olivier KN, Milla C, Daniel SJ, Kimple AJ, Manion M, Knowles MR, Leigh MW; Genetic Disorders of Mucociliary Clearance Consortium. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol. 2016 Feb;51(2):115-32. doi: 10.1002/ppul.23304. PMID: 26418604; PMCID: PMC8835943.
- Corvol H, Blackman SM, Boëlle PY, Gallins PJ, Pace RG, Stonebraker JR, Accurso FJ, Clement A, Collaco JM, Dang H, Dang AT, Franca A, Gong J, Guillot L, Keenan K, Li W, Lin F, Patrone MV, Raraigh KS, Sun L, Zhou YH, O’Neal WK, Sontag MK, Levy H, Durie PR, Rommens JM, Drumm ML, Wright FA, Strug LJ, Cutting GR, Knowles MR. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat Commun. 2015 Sep 29;6:8382. doi: 10.1038/ncomms9382. PMID: 26417704; PMCID: PMC4589222.
- O’Neal WK, Gallins P, Pace RG, Dang H, Wolf WE, Jones LC, Guo X, Zhou YH, Madar V, Huang J, Liang L, Moffatt MF, Cutting GR, Drumm ML, Rommens JM, Strug LJ, Sun W, Stonebraker JR, Wright FA, Knowles MR. Gene expression in transformed lymphocytes reveals variation in endomembrane and HLA pathways modifying cystic fibrosis pulmonary phenotypes. Am J Hum Genet. 2015 Feb 5;96(2):318-28. doi: 10.1016/j.ajhg.2014.12.022. PMID: 25640674; PMCID: PMC4320265.
- Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, Sagel SD, Milla C, Zariwala MA, Pittman JE, Shapiro AJ, Carson JL, Krischer JP, Hazucha MJ, Cooper ML, Knowles MR, Leigh MW. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015 Feb 1;191(3):316-24. doi: 10.1164/rccm.201409-1672OC. PMID: 25493340; PMCID: PMC4351577.
- Lucas JS, Behan L, Dunn Galvin A, Alpern A, Morris AM, Carroll MP, Knowles MR, Leigh MW, Quittner AL. A quality-of-life measure for adults with primary ciliary dyskinesia: QOL-PCD. Eur Respir J. 2015 Aug;46(2):375-83. doi: 10.1183/09031936.00216214. PMID: 25976687; PMCID: PMC4522020.
- Lin J, Yin W, Smith MC, Song K, Leigh MW, Zariwala MA, Knowles MR, Ostrowski LE, Nicastro D. Cryo-electron tomography reveals ciliary defects underlying human RSPH1 primary ciliary dyskinesia. Nat Commun. 2014 Dec 4;5:5727. doi: 10.1038/ncomms6727. PMID: 25473808; PMCID: PMC4267722
- Daniels ML, Lowe JR, Roy P, Patrone MV, Conyers JM, Fine JP, Knowles MR, Birchard KR. Standardization and validation of a novel and simple method to assess lumbar dural sac size. Clin Radiol. 2015 Feb;70(2):146-52. doi: 10.1016/j.crad.2014.10.009. PMID: 25434773; PMCID: PMC4282821.
- Prevots DR, Adjemian J, Fernandez AG, Knowles MR, Olivier KN. Environmental risks for nontuberculous mycobacteria. Individual exposures and climatic factors in the cystic fibrosis population. Ann Am Thorac Soc. 2014 Sep;11(7):1032-8. doi: 10.1513/AnnalsATS.201404-184OC. PMID: 25068620; PMCID: PMC4214058.
- Shapiro AJ, Weck KE, Chao KC, Rosenfeld M, Nygren AO, Knowles MR, Leigh MW, Zariwala MA. Cri du chat syndrome and primary ciliary dyskinesia: a common genetic cause on chromosome 5p. J Pediatr. 2014 Oct;165(4):858-61. doi: 10.1016/j.jpeds.2014.06.048. PMID: 25066065; PMCID: PMC4177261.
- Shapiro AJ, Davis SD, Ferkol T, Dell SD, Rosenfeld M, Olivier KN, Sagel SD, Milla C, Zariwala MA, Wolf W, Carson JL, Hazucha MJ, Burns K, Robinson B, Knowles MR, Leigh MW. Laterality defects other than situs inversus totalis in primary ciliary dyskinesia: insights into situs ambiguus and heterotaxy. Chest. 2014 Nov;146(5):1176-86. doi: 10.1378/chest.13-1704. PMID: 24577564; PMCID: PMC4219335.
- Knowles MR, Ostrowski LE, Leigh MW, Sears PR, Davis SD, Wolf WE, Hazucha MJ, Carson JL, Olivier KN, Sagel SD, Rosenfeld M, Ferkol TW, Dell SD, Milla CE, Randell SH, Yin W, Sannuti A, Metjian HM, Noone PG, Noone PJ, Olson CA, Patrone MV, Dang H, Lee HS, Hurd TW, Gee HY, Otto EA, Halbritter J, Kohl S, Kircher M, Krischer J, Bamshad MJ, Nickerson DA, Hildebrandt F, Shendure J, Zariwala MA. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med. 2014 Mar 15;189(6):707-17. doi: 10.1164/rccm.201311-2047OC. PMID: 24568568; PMCID: PMC3983840.
- Kim RH, A Hall D, Cutz E, Knowles MR, Nelligan KA, Nykamp K, Zariwala MA, Dell SD. The role of molecular genetic analysis in the diagnosis of primary ciliary dyskinesia. Ann Am Thorac Soc. 2014 Mar;11(3):351-9. doi: 10.1513/AnnalsATS.201306-194OC. PMID: 24498942; PMCID: PMC4028737.
- Knowles MR, Ostrowski LE, Loges NT, Hurd T, Leigh MW, Huang L, Wolf WE, Carson JL, Hazucha MJ, Yin W, Davis SD, Dell SD, Ferkol TW, Sagel SD, Olivier KN, Jahnke C, Olbrich H, Werner C, Raidt J, Wallmeier J, Pennekamp P, Dougherty GW, Hjeij R, Gee HY, Otto EA, Halbritter J, Chaki M, Diaz KA, Braun DA, Porath JD, Schueler M, Baktai G, Griese M, Turner EH, Lewis AP, Bamshad MJ, Nickerson DA, Hildebrandt F, Shendure J, Omran H, Zariwala MA.Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am J Hum Genet. 2013 Oct 3;93(4):711-20. doi: 10.1016/j.ajhg.2013.07.025. PMID: 24055112;
- Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, Lavange LM, Horton BJ, Qaqish B, Carson JL, Davis SD, Dell SD, Ferkol TW, Atkinson JJ, Olivier KN, Sagel SD, Rosenfeld M, Milla C, Lee HS, Krischer J, Zariwala MA, Knowles MR. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013 Dec;10(6):574-81. doi: 10.1513/AnnalsATS.201305-110OC. PMID: 24024753; PMCID: PMC3960971.
- Daniels ML, Leigh MW, Davis SD, Armstrong MC, Carson JL, Hazucha M, Dell SD, Eriksson M, Collins FS, Knowles MR, Zariwala MA. Founder mutation in RSPH4A identified in patients of Hispanic descent with primary ciliary dyskinesia. Hum Mutat. 2013 Oct;34(10):1352-6. doi: 10.1002/humu.22371. PMID: 23798057; PMCID: PMC3906677.
- Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013 Oct 15;188(8):913-22. doi: 10.1164/rccm.201301-0059CI. Review. PMID: 23796196; PMCID: PMC3826280.
- Knowles MR, Leigh MW, Ostrowski LE, Huang L, Carson JL, Hazucha MJ, Yin W, Berg JS, Davis SD, Dell SD, Ferkol TW, Rosenfeld M, Sagel SD, Milla CE, Olivier KN, Turner EH, Lewis AP, Bamshad MJ, Nickerson DA, Shendure J, Zariwala MA; Genetic Disorders of Mucociliary Clearance Consortium. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013 Jan 10;92(1):99-106. doi: 10.1016/j.ajhg.2012.11.003. PMID: 23261302; PMCID: PMC3542458.
- Knowles MR, Leigh MW, Carson JL, Davis SD, Dell SD, Ferkol TW, Olivier KN, Sagel SD, Rosenfeld M, Burns KA, Minnix SL, Armstrong MC, Lori A, Hazucha MJ, Loges NT, Olbrich H, Becker-Heck A, Schmidts M, Werner C, Omran H, Zariwala MA; Genetic Disorders of Mucociliary Clearance Consortium. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 2012 May;67(5):433-41. doi: 10.1136/thoraxjnl-2011-200301. PMID: 22184204; PMCID: PMC3739700.
