The major areas of research are organized thematically around the following topics; each tab when clicked will reveal more details on each related project.
- ANCA Vasculitis and Systemic Lupus Erythematosus
- Glomerular Diseases
- Diabetic Kidney Disease
- Epidemiology and Outcomes Research in Kidney Disease
- Kidney Development and Function
- Novel Imaging Techniques in Nephrology
ANCA Vasculitis and Systemic Lupus Erythematosus
This research is a successful collaboration between basic and translational scientists, epidemiologists, geneticists, and nephropathologists. The themes of the projects mirror questions that patients frequently ask: what causes their disease, what will make their disease better or worse, and what will assure that their disease goes into remission and even be cured? Dr. Ronald Falk is Principal Investigator.
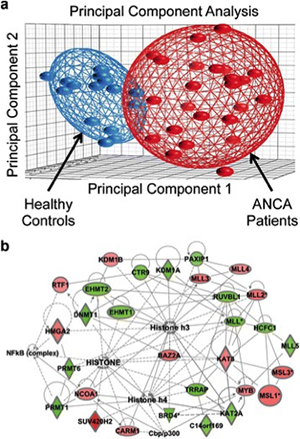
This project aims at unraveling the molecular underpinning of ANCA GN by elucidating genetic variation, epigenetic regulation of transcription, and clarification of the transcriptional profiles of the inflammatory cells engaged in the pathogenesis of ANCA vasculitis (neutrophils, monocytes, lymphocytes and Myeloid Derived Suppressor Cells [MDSC]). The Investigation team is led by Dominic Ciavatta, PhD and includes Ronald Falk, MD, Meghan Free, PhD, J. Charles Jennette, MD and Terry Magnuson, PhD, William Pendergraft, MD, PhD and Joshua Starmer, PhD in the Department of Genetics.
Recent studies have focused on identifying HLA variants of ANCA GN among our patient cohort and use the corresponding HLA to identify autoreactive T cells. Initial analysis of the North American GWAS sequenced HLA from over 450 patients revealed that the strongest association among ANCA GN was found for HLA-DPB1,. HLA sequencing data of our ANCA-GN patient cohort identified the two most common HLA as DPB1*04:01 (65.6% carrier frequency) and DRB4*01:01 (59.4% carrier frequency), (Arthritis Rheumatol 2017; 69(5):1054-66). In collaboration with Bjoern Peters at La Jolla Institute for Allergy and Immunology, PR3 and MPO peptides were identified that bind DPB1*04:01 and DRB4*01:01 and further studies were performed with patients carrying DPB1*04:01 or DRB4*01:01 to isolate autoreactive T cells that recognize MPO peptides. The exciting result of these studies demonstrated that patients with ANCA GN have B and T cell responses to overlapping MPO epitopes.
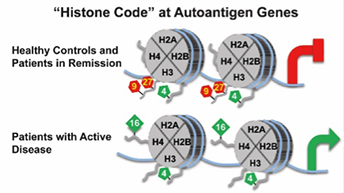
In relates studies, it was determined that MPO and PRTN3 in neutrophils of patients with ANCA-associated vasculitis with active disease have a distinct pattern of histone modifications, which implicates epigenetic mechanisms in regulating expression of autoantigen genes. While additional questions remain to be answered, the close association of specific histone modifications with active AAV is consistent with the hypothesis that an epigenetic signature corresponds to disease status, and suggests that a dynamic epigenome is involved in the pathogenesis of AAV. In addition to the presence of autoantibodies, the plasticity of the epigenome may promote the development of AAV (Clin Epigenetics 2016; 8:85 eCollection).
T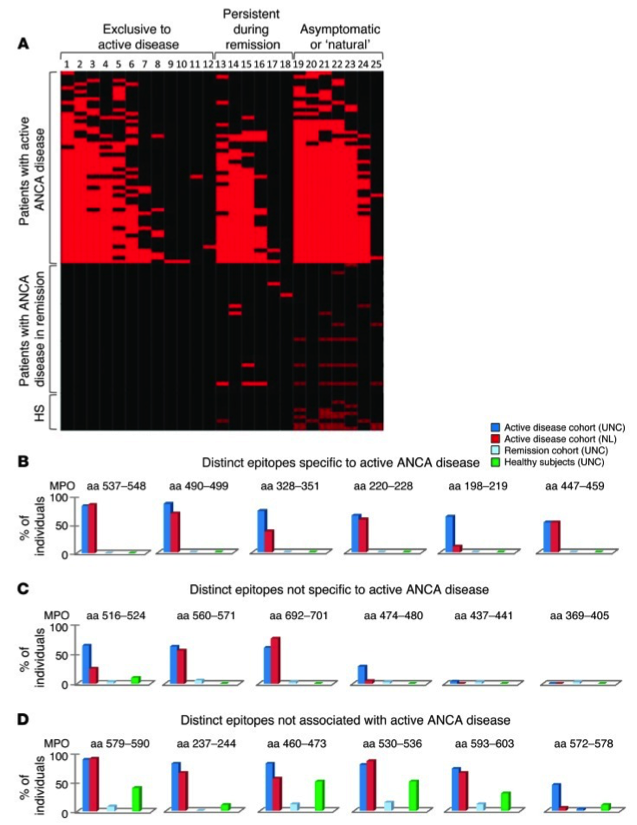 his project seeks to identify immunodominant ANCA autoantigens using state-of-the-art mass spectrometry and understand the modulation of adaptive immune responses on the risk and severity of ANCA GN. The investigation team is led by Ronald Falk, MD and includes Donna Bunch, PhD and Meghan Free, PhD.
his project seeks to identify immunodominant ANCA autoantigens using state-of-the-art mass spectrometry and understand the modulation of adaptive immune responses on the risk and severity of ANCA GN. The investigation team is led by Ronald Falk, MD and includes Donna Bunch, PhD and Meghan Free, PhD.
Recent studies have built extensively on findings by Falk and colleagues of an immunodominant linear epitope of MPO. Utilizing an in-house ELISA directed against this linear epitope, it was learned that antibodies to this epitope are most detectable at onset of disease, and in some patients correlate with disease activity. The combination of B cells studies and HLA sequencing made it possible to create tetramers to study autoreactive T cells in the periphery of ANCA vasculitis patients.
To investigate the targets of autoantibodies in drug-associated ANCA vasculitis compared to idiopathic disease, a new immunosignature platform was employed composed of a random-sequence peptide library, and novel patterns associated with disease activity (active vs. remission) and drug-associated disease were identified. The peptides and corresponding proteins are being further interrogated with GuiTope analysis to predict autoantibody target epitope(s) and new binding partners to better discriminate ANCA subgroups.
This project investigates the adaptive and innate immune responses that influence the risk, severity and clinicopathologic phenotypes of ANCA glomerulonephritis. The investigation team is led by J. Charles Jennette, MD and includes Donna Bunch, PhD and Dominic Ciavatta, PhD, Peiqi Hu, MD and Hong Xiao, MD.
A landmark in ANCA research has been the development of a mouse model that reproduces the human histopathology and definitively demonstrated that anti-MPO antibodies are indeed pathogenic. This mouse model allows the role of various inflammatory pathways in the pathogenesis of ANCA vasculitis. Through a series of experiments in mice deficient in various components of the complement cascade, this model identified an important role of the alternative pathway of complement, but not the terminal membrane attack complex, in the pathogenesis of anti-MPO mediated disease. This work is the basis upon which novel therapeutic approaches for human ANCA vasculitis are being developed.
A substantial achievement is the ability to now produce granulomatosis in mice that looks nearly identical to human granulomatosis with polyangiitis. This group has also developed an experimental technique that allows monitoring of MPO-ANCA pulmonary disease while the mice are alive, facilitating the development of translational preclinical models to be used to determine the efficacy of treatment in ANCA pulmonary disease. New ground was recently broken by identifying mediator systems that have not been previously incriminated in ANCA-GN pathogenesis. The recently developed mouse model will be used to investigate epigenetic regulation of neutrophil autoantigen gene expression as a risk factor for and modulator of ANCA GN. Studies are currently being conducted to determine the role of complement and complement regulatory proteins in the in vivo model of MPO-ANCA GN, and have evaluated the effects of complement activation on the new GPA pulmonary granulomatosis model. Early results indicate that the mediators for necrotizing GN and necrotizing pulmonary granulomatosis are different, and optimum therapy for kidney disease may differ from optimum therapy for lung disease.
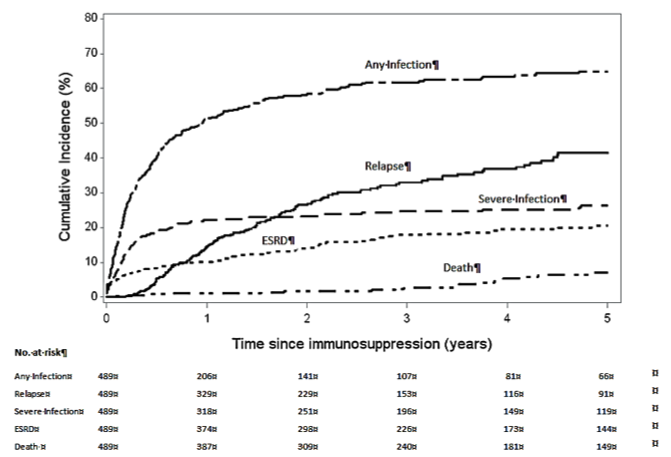 This investigation team is led by Susan Hogan, PhD, MPH and Patrick Nachman, MD and includes Donna Bunch, PhD, Ronald Falk, MD, J. Charles Jennette, MD, William Pendergraft and Caroline Poulton, MSW. This project investigates clinical applications to maintain durable remission, minimize relapse and prevent infection and ANCA GN. The last decades have witnessed great progress in the induction therapy of ANCA GN and vasculitis, resulting in an improvement in outcomes from 80% mortality to 80% induction of remission. This success has resulted in a shift in the main cause of mortality from the underlying vascular inflammation and disease to that of complications of immunosuppressive therapy, and most commonly infectious complications. Minimizing those risks and the frequency of complication resulting from therapy is being addressed in these studies through a 3-pronged approach. The first step is to minimize the infections complications of therapy by identifying the serologic and molecular characteristics of patients who are in complete remission and a low risk of relapse in whom maintenance immunotherapy may be discontinued. The second approach uses a pilot clinical trial to test the feasibility of tailoring maintenance immunosuppression based on the regulatory B cells after induction therapy. For patients who require induction or maintenance immunosuppressive therapy, prophylactic antibiotics are being tested for the prevention of infectious complications. A retrospective analysis of the UNC large registry of patients with ANCA vasculitis has allowed to identify patients who have attained complete remission off of immunosuppressive therapy and maintained it for 1 year, 2 years or more than 5 years. The probability of relapse after 2 years of completion remission off therapy is significantly lower than for patients who have not attained this landmark. Analysis of the clinical characteristics of patients who have attained a long-term remission off therapy has allowed significant progress in establishing methods for testing various molecular, epigenetic and serologic biomarkers of stable remission. The overarching goal of this work is to be able to identify prospectively patients in remission who would not need to remain on maintenance immunotherapy. A proof of concept trial using B cell phenotyping to this end is under development.
This investigation team is led by Susan Hogan, PhD, MPH and Patrick Nachman, MD and includes Donna Bunch, PhD, Ronald Falk, MD, J. Charles Jennette, MD, William Pendergraft and Caroline Poulton, MSW. This project investigates clinical applications to maintain durable remission, minimize relapse and prevent infection and ANCA GN. The last decades have witnessed great progress in the induction therapy of ANCA GN and vasculitis, resulting in an improvement in outcomes from 80% mortality to 80% induction of remission. This success has resulted in a shift in the main cause of mortality from the underlying vascular inflammation and disease to that of complications of immunosuppressive therapy, and most commonly infectious complications. Minimizing those risks and the frequency of complication resulting from therapy is being addressed in these studies through a 3-pronged approach. The first step is to minimize the infections complications of therapy by identifying the serologic and molecular characteristics of patients who are in complete remission and a low risk of relapse in whom maintenance immunotherapy may be discontinued. The second approach uses a pilot clinical trial to test the feasibility of tailoring maintenance immunosuppression based on the regulatory B cells after induction therapy. For patients who require induction or maintenance immunosuppressive therapy, prophylactic antibiotics are being tested for the prevention of infectious complications. A retrospective analysis of the UNC large registry of patients with ANCA vasculitis has allowed to identify patients who have attained complete remission off of immunosuppressive therapy and maintained it for 1 year, 2 years or more than 5 years. The probability of relapse after 2 years of completion remission off therapy is significantly lower than for patients who have not attained this landmark. Analysis of the clinical characteristics of patients who have attained a long-term remission off therapy has allowed significant progress in establishing methods for testing various molecular, epigenetic and serologic biomarkers of stable remission. The overarching goal of this work is to be able to identify prospectively patients in remission who would not need to remain on maintenance immunotherapy. A proof of concept trial using B cell phenotyping to this end is under development.
 Susan Hogan, PhD, MPH is an Associate Professor in the UNC Kidney Center. She is the lead epidemiologist for the Center. She has coordinated the research efforts of the Glomerular Disease Collaborative Network (GDCN) and has been extensively involved in studies of vascular and glomerular diseases, dialysis, and transplantation encompassing a very broad spectrum of renal diseases. She utilizes basic and advanced statistical methods to interpret variable data and has participated or led cohort studies of various glomerular diseases and population-based control studies in lupus nephritis, membranous glomerulopathy and vasculitis. An important research study was undertaken to evaluate the applicability of predictors of treatment resistance and relapse in patients with ANCA-associated vasculitis in the GDCN. This important study identified an increased risk for relapse related to the presence of lung or upper airway disease and anti-PR3 antibody seropositivity, and laid early groundwork for later studies that have established phenotypic, genetic and clinical differences between PR3- and MPO-ANCA disease. The UNC/GDCN ANCA disease registry currently includes more than 2,1 patients Dr Hogan’s recent research has focused on pesticide exposure and end-stage kidney disease as part of the Agricultural Health Study. In a study of male pesticide applicators, risk of end-stage kidney disease increased with increasing cumulative exposure to several pesticides and the risk was significantly greater for pesticide applicators who reported multiple doctor visits and hospitalization due to pesticide use (Occup Environ Med 2016; 73(1):3-12).
Susan Hogan, PhD, MPH is an Associate Professor in the UNC Kidney Center. She is the lead epidemiologist for the Center. She has coordinated the research efforts of the Glomerular Disease Collaborative Network (GDCN) and has been extensively involved in studies of vascular and glomerular diseases, dialysis, and transplantation encompassing a very broad spectrum of renal diseases. She utilizes basic and advanced statistical methods to interpret variable data and has participated or led cohort studies of various glomerular diseases and population-based control studies in lupus nephritis, membranous glomerulopathy and vasculitis. An important research study was undertaken to evaluate the applicability of predictors of treatment resistance and relapse in patients with ANCA-associated vasculitis in the GDCN. This important study identified an increased risk for relapse related to the presence of lung or upper airway disease and anti-PR3 antibody seropositivity, and laid early groundwork for later studies that have established phenotypic, genetic and clinical differences between PR3- and MPO-ANCA disease. The UNC/GDCN ANCA disease registry currently includes more than 2,1 patients Dr Hogan’s recent research has focused on pesticide exposure and end-stage kidney disease as part of the Agricultural Health Study. In a study of male pesticide applicators, risk of end-stage kidney disease increased with increasing cumulative exposure to several pesticides and the risk was significantly greater for pesticide applicators who reported multiple doctor visits and hospitalization due to pesticide use (Occup Environ Med 2016; 73(1):3-12).
Will Pendergraft III, MD, PhD specializes in autoimmune glomerular disease and vasculitis. His clinical and translational research revolves around understanding the immunopathogenetic mechanisms underlying lupus nephritis and ANCA vasculitis.
Dr. Pendergraft is engaged in building a world-renowned lupus nephritis center here at UNC and has formed the Chapel Hill Alliance to Promote Excellence in Lupus (CHAPEL for short), which has patient care, education, research and clinical trial arms. The flagship product of CHAPEL is the Systems-Level Temporal Observation of Patients with Lupus (STOP SLE) study, which is the first of its kind study and is funded by a consortium between two pharmaceutical companies and in collaboration with The Broad Institute. STOP SLE recruits participants from the UNC Health Care system and uses high-tech and cutting-edge tools to identify predictors or biomarkers of disease and treatment response in patients with newly-diagnosed lupus nephritis. Dr. Pendergraft also directs all of the clinical trials at UNC focused on lupus nephritis, of which UNC has a large population, and his laboratory is engaged in clinical research seeking to understand rare variants of lupus nephritis as well as other orphan glomerular diseases. He collaborates in translational research in ANCA vasculitis under a long-standing NIDDK-funded program project grant with his main clinical mentor, Dr. Ronald Falk.
Interestingly, Dr. Pendergraft has also established a strong collaboration with the Carolinas Poison Control Center and its medical director, Dr. Michael Beuhler, to analyze exposures to nephrotoxins across the United States. The clinical aspects and outcomes of antifreeze exposures were recently published.
Diabetic Kidney Disease
 Amy Mottl, MD, MPH investigates factors that affect progression of diabetic kidney disease. People with diabetes vary greatly in the rate at which they progress to needing dialysis but the reasons for this are unclear. In a collaboration with Dr. J. Charles Jennette (Chair of Pathology and Laboratory Medicine), they discovered that some people have signs of particularly severe injury to one of the key cell types in the kidney (podocytes), and that people with these changes progress extremely rapidly to end-stage kidney disease. They are studying the molecular changes found in the tissue of patients with these findings, which could lead to new methods to risk stratify patients with diabetic kidney disease.
Amy Mottl, MD, MPH investigates factors that affect progression of diabetic kidney disease. People with diabetes vary greatly in the rate at which they progress to needing dialysis but the reasons for this are unclear. In a collaboration with Dr. J. Charles Jennette (Chair of Pathology and Laboratory Medicine), they discovered that some people have signs of particularly severe injury to one of the key cell types in the kidney (podocytes), and that people with these changes progress extremely rapidly to end-stage kidney disease. They are studying the molecular changes found in the tissue of patients with these findings, which could lead to new methods to risk stratify patients with diabetic kidney disease.
Dr. Mottl is also the UNC PI for the TRIDENT Study (Transformative Research in DiabEtic NephropaThy), which is a multicenter study to prospectively studying patients with diabetes undergoing kidney biopsy. Participants will be followed for three years following biopsy to determine how quickly their kidney disease progresses. Kidney tissue and other biologic samples will be studied to identify molecules that are associated with more rapid progression of disease, in order to identify risk markers and new targets for treatment of diabetic kidney disease.
Epidemiology and Outcomes Research in Kidney Disease
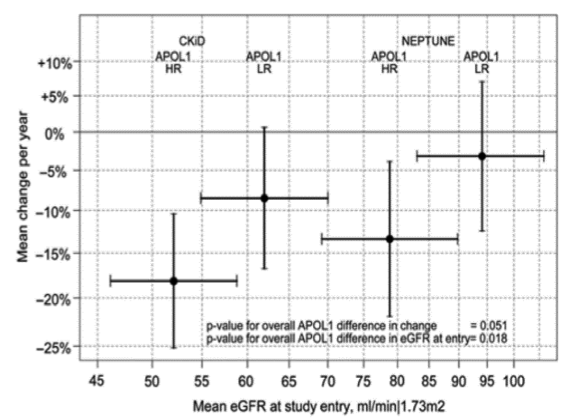
Keisha Gibson, MD, MPH is an Assistant Professor in the UNC Kidney Center. Her research is focused on understanding the biologic and non-biologic determinants of outcome disparities in African Americans with glomerular disease. Specifically she is examining the genotypic and clinical predictors of treatment response and long-term renal survival in African Americans affected by glomerular disease. Recent attention has focused on the rapid progression of end stage kidney disease (ESKD) in African Americans with two copies of the APOL1 risk alleles, especially in a subset of African Americans with this APOL1 high-risk allele genotype who do poorly. It is estimated that up to 11% of African Americans carry this high-risk genotype and while there certainly appears to be an association with poor outcome, there is no data examining what may protect those with this high-risk background from either developing kidney disease or progressing to ESKD. Using whole exome sequencing, Dr. Gibson is working to identify novel risk alleles by comparing exomes from African American patients with glomerular disease that do not have kidney disease, respond favorably to treatment and/or have good long-term renal survival compared to those who respond poorly and/or have progressive renal failure. Dr. Gibson has worked to expand a registry and biorepository of DNA, RNA, and sera of both adult and pediatric patients with lupus nephritis and nephrotic syndromes under the umbrella of the Glomerular Disease Collaborative Network (GDCN). The GDCN is a collaborative network of over 300 physicians and the registry includes data from over 5000 patients with glomerular disease.
Dr. Gibson is Chair for the Pediatric Studies Committee and a site Co-Investigator with Drs. Patrick Nachman and Susan Hogan for the multi-site Nephrotic Syndrome Study Network (NEPTUNE) as part of the NIH Rare Diseases Clinical Research Network. The purpose of this study is to gather long-term observational data in order to help understand the biology behind the nephrotic syndrome. She is also the Pediatric Site Investigator for CureGN. She was recently selected for the Simmons Scholars Career Development Program, which is supporting her study of the genetic predisposition to end-stage kidney disease in African Americans with glomerular disease. UNC is a leading recruitment site, with over 250 children and 600 adults with nephrotic syndrome currently enrolled in this study. By integrating molecular and clinical data in this well characterized cohort, this study aims to enhance the understanding the pathogenesis of these rare diseases that present as a common histological phenotype and advance innovation leading towards a precision medicine approach to treatment.
 Abhi Kshirsagar, MD, MPH is engaged almost exclusively to health services related research in dialysis. He had a significant role in a grant sponsored by the Agency for Healthcare Quality and Research pertaining to the safety and effectiveness of intravenous iron administration in the dialysis population. Another research project is investigating the comparative effectiveness and safety of intravenous iron in the dialysis population. As the clinical Co-Investigator of this project with Drs. Alan Brookhart and Til Stürmer of the Gillings School of Global Public Health, he is providing the medical context for this long-term epidemiology study. He is also in the process of developing and validating a screening tool for chronic kidney disease and obtained an R03 grant to test the effectiveness of his screening tool in rural counties in North Carolina, which forms the basis of an R34 application on kidney education that is now in development.
Abhi Kshirsagar, MD, MPH is engaged almost exclusively to health services related research in dialysis. He had a significant role in a grant sponsored by the Agency for Healthcare Quality and Research pertaining to the safety and effectiveness of intravenous iron administration in the dialysis population. Another research project is investigating the comparative effectiveness and safety of intravenous iron in the dialysis population. As the clinical Co-Investigator of this project with Drs. Alan Brookhart and Til Stürmer of the Gillings School of Global Public Health, he is providing the medical context for this long-term epidemiology study. He is also in the process of developing and validating a screening tool for chronic kidney disease and obtained an R03 grant to test the effectiveness of his screening tool in rural counties in North Carolina, which forms the basis of an R34 application on kidney education that is now in development.
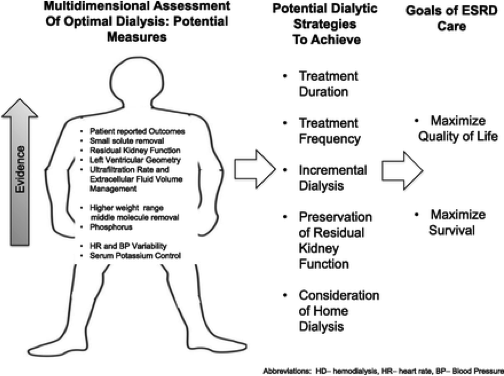 Jenny Flythe, MD, MPH is a nephrologist and clinician-scientist who in the Principal Investigator of a dialysis research program currently funded by NIH/NIDDK, PCORI, and industry. She conducts epidemiologic, qualitative and clinical trial studies designed to inform dialysis clinical practice. Her primary areas of expertise are in two major areas: (1) fluid management and blood pressure, and (2) patient-reported outcomes. Through epidemiologic investigations, Dr. Flythe has established an association between more rapid fluid removal (ultrafiltration) during dialysis and adverse cardiovascular outcomes. This seminal work was the basis for a recently approved clinical quality measure for the Centers for Medicare and Medicaid Services (CMS) End Stage Renal Disease Quality Incentive Program, a pay-for-performance Medicare program. Curbing harm from fluid-related practices now considered one of the most pressing clinical challenges in dialysis, and all major dialysis providers have implemented ultrafiltration rate clinical policies centered upon Dr. Flythe’s work. Her fluid-related research is funded by an NIH/NIDDK K23 Career Development Award. Under the award, she is investigating the association of diuretic cessation and cardiovascular-outcomes among individuals initiating dialysis via an epidemiologic study and performing a double-blinded, cross-over clinical trial investigating the effect of ultrafiltration profiling on cardiovascular outcomes.
Jenny Flythe, MD, MPH is a nephrologist and clinician-scientist who in the Principal Investigator of a dialysis research program currently funded by NIH/NIDDK, PCORI, and industry. She conducts epidemiologic, qualitative and clinical trial studies designed to inform dialysis clinical practice. Her primary areas of expertise are in two major areas: (1) fluid management and blood pressure, and (2) patient-reported outcomes. Through epidemiologic investigations, Dr. Flythe has established an association between more rapid fluid removal (ultrafiltration) during dialysis and adverse cardiovascular outcomes. This seminal work was the basis for a recently approved clinical quality measure for the Centers for Medicare and Medicaid Services (CMS) End Stage Renal Disease Quality Incentive Program, a pay-for-performance Medicare program. Curbing harm from fluid-related practices now considered one of the most pressing clinical challenges in dialysis, and all major dialysis providers have implemented ultrafiltration rate clinical policies centered upon Dr. Flythe’s work. Her fluid-related research is funded by an NIH/NIDDK K23 Career Development Award. Under the award, she is investigating the association of diuretic cessation and cardiovascular-outcomes among individuals initiating dialysis via an epidemiologic study and performing a double-blinded, cross-over clinical trial investigating the effect of ultrafiltration profiling on cardiovascular outcomes.
Dr. Flythe’s second area of research expertise is in patient-reported outcomes and patient-engagement. She is conducting a PCORI-funded project aimed at driving better research engagement of stakeholders within local dialysis clinics. Through nationwide collaborations with stakeholders, the team is developing dialysis-specific research materials for patients and dialysis clinic staff. Through an industry-awarded grant, she is working collaboratively with the two largest dialysis provider organizations to develop a point-of-care symptom assessment tool for individuals on in-center hemodialysis. She serves as the co-chairperson of a Kidney Health Initiative (public-private partnership between the American Society of Nephrology (ASN) and the Food and Drug Administration) project entitled, “Prioritizing Symptoms of ESRD Patients.” The group is spearheading a national iterative, consensus-building effort to develop a roadmap for innovation in symptom management among dialysis patients. She is also the co-chairperson of an ongoing CMS Technical Expert Panel tasked with developing recommendations for a patient-reported outcome clinical quality measure for agency use in dialysis-related programs.
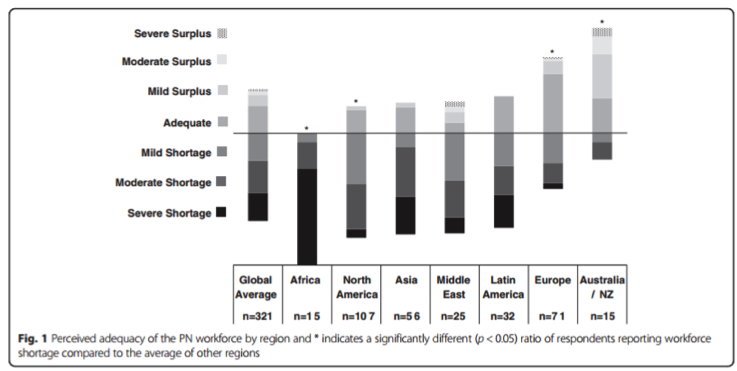 Dorey Glenn, MD is a pediatric nephrologist who researches the epidemiology of adverse outcomes in patients with glomerular disease. His current work includes assessing rates of skeletal fracture in UNC’s Glomerular Disease Collaborative Network cohort and analyzing infectious complications in a national cohort of patients with glomerular disease. Dr. Glenn has explored his interest in global health by assessing the perceived adequacy of the international pediatric nephrology workforce and supporting nephrology care in the Department of Pediatrics at the National University of Bhutan. He is currently involved in a project creating a decision support tool to aid physicians with management of patients with acute glomerulonephritis in resource-limited settings (BMC Nephrol 2016; 17:83).
Dorey Glenn, MD is a pediatric nephrologist who researches the epidemiology of adverse outcomes in patients with glomerular disease. His current work includes assessing rates of skeletal fracture in UNC’s Glomerular Disease Collaborative Network cohort and analyzing infectious complications in a national cohort of patients with glomerular disease. Dr. Glenn has explored his interest in global health by assessing the perceived adequacy of the international pediatric nephrology workforce and supporting nephrology care in the Department of Pediatrics at the National University of Bhutan. He is currently involved in a project creating a decision support tool to aid physicians with management of patients with acute glomerulonephritis in resource-limited settings (BMC Nephrol 2016; 17:83).Kidney Development and Function
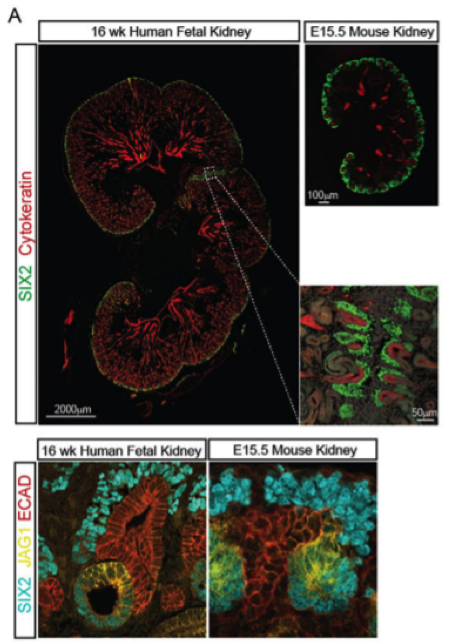
Lori O’Brien, PhD is Assistant Professor in the Department of Cell Biology and Physiology and a member of the UNC Kidney Center. Her work focuses on several aspects of kidney development as well as a highly specialized cell of the glomerular filter, namely the podocyte. By understanding kidney development and how progenitor cells are maintained and differentiated, we can translate these findings to congenital diseases and pediatric tumors of the kidney where abnormal regulation of these progenitor cells are often the culprit. In addition, our understanding of kidney development will aid in the development of regenerative strategies to treat kidney disease. Dr. O’Brien is specifically interested in signaling molecules and transcription factors which regulate kidney progenitor cells.
Podocytes are an essential component of the glomerular filter and their compromise is associated with kidney disease. Dr. O’Brien has studied how these cells form during development and is interested in how they change during disease and aging. Specifically, she is working to identify epigenetic changes which occur in podocytes during these processes and whether modulating these changes can help restore or rejuvenate these cells.
Prevalence rates of premature births are rising in the United States. In 2015, the Centers for Disease Control (CDC) reported that approximately 1 in 10 infants were born premature in the US. With the advancements in perinatal-neonatal medicine, approximately 90% of infants born premature survive to neonatal intensive care unit (NICU) discharge. However, approximately 60-80% of human nephrons (functional units of the kidney) are formed during the second and third trimesters of gestation. Preterm infants are often born during this active period of kidney formation halting their normal development. This leads to a reduction in total nephron numbers which is thought to increase the risk for the development of chronic kidney disease later in life, which itself is associated with a 100-fold greater risk of cardiovascular mortality than the healthy general population.
While the pulmonary and neurodevelopmental consequences of prematurity are well under surveillance, the renal consequences of prematurity and the associations with perinatal comorbidities in children are not as well studied. In a sample of children with a history of extremely low gestational age (born before 28 weeks gestation), Dr. Keia Sanderson is evaluating these children for early evidence of kidney disease during childhood and investigating for associations between early life exposures (medications received by the infant and/or prenatal and postnatal co-morbidities) and kidney disease.
Novel Imaging Techniques
 Contrast-enhanced ultrasound (CEUS) is an emerging imaging technique which is gaining popularity due to its excellent side effect profile and its lack of toxic effects in patients with kidney disease. This technique utilizes the use of microbubbles as contrast agents. Microbubbles, unlike gadolinium-based or iodinated contrast agents, do not extravasate out of vessels, making it a more accurate tool to detect perfusion. In collaboration with Dr. Kim Rathmell, Wui Chong and Paul Dayton, Dr. Emily Chang has recently submitted results of a pilot study investigating the use of CEUS in patients with and without kidney disease. Results suggest that CEUS has high sensitivity in patients with and without kidney disease when classifying a kidney lesion as malignant or benign, comparable to contrast-enhanced CT in the general population. Based on these results, the collaborative team designed a second study targeting only patients with kidney disease, which is currently in the follow-up phase. This study is being funded by the Improving Human Health Grant by NC TraCS.
Contrast-enhanced ultrasound (CEUS) is an emerging imaging technique which is gaining popularity due to its excellent side effect profile and its lack of toxic effects in patients with kidney disease. This technique utilizes the use of microbubbles as contrast agents. Microbubbles, unlike gadolinium-based or iodinated contrast agents, do not extravasate out of vessels, making it a more accurate tool to detect perfusion. In collaboration with Dr. Kim Rathmell, Wui Chong and Paul Dayton, Dr. Emily Chang has recently submitted results of a pilot study investigating the use of CEUS in patients with and without kidney disease. Results suggest that CEUS has high sensitivity in patients with and without kidney disease when classifying a kidney lesion as malignant or benign, comparable to contrast-enhanced CT in the general population. Based on these results, the collaborative team designed a second study targeting only patients with kidney disease, which is currently in the follow-up phase. This study is being funded by the Improving Human Health Grant by NC TraCS.
In addition to investigating the accuracy of CEUS for characterizing kidney lesions, Dr. Chang is also investigating the use of CEUS for other applications in patients with kidney disease, including patients with kidney transplant. She also works collaboratively with the laboratory of Dr. Paul Dayton to conduct pre-clinical studies aimed at understanding the properties of CEUS better in animal models so that they can be interpreted more accurately in humans.