The Zylka and the Jewells groups look at neuroinflammation with PBR111 PET imaging on animal models of neurological diseases.
The peripheral benzodiazepine receptor (PBR), or 18-kDa translocator protein (TSPO), is shown to be essential for cholesterol transport and steroid hormone production [1]. Unlike the central benzodiazepine receptor (CBR), the PBR is a mitochondrial protein found in both brain (mainly glial cells) and peripheral tissues (adrenal gland, heart, lung, kidney, and testis) [2]. In normal conditions, the brain has lower levels of PBR than do peripheral tissues. Increased PBR expression has been found in brain injuries or neuroinflammatory conditions where microglia cells are activated as an inflammatory response [3]. Increased PBR levels have also been reported in several neurodegenerative diseases, including Alzheimer’s disease, Wernicke’s encephalopathy, multiple sclerosis, and epilepsy [4].
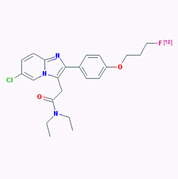
The high expression of PBR in neuroinflammation and neurodegenerative diseases suggests that the PBR could be a clinically useful marker for the detection of these disorders. Several PBR ligands have been developed to target PBR, including agent PK11195 and PBR111, both of which can be labeled with radioisotopes, either C-11 or F-18, for PET imaging. Fookes et al in 2008 first reported the synthesis and biodistribution of F-18 labeled PBR111 as a PET imaging agent [5]. The molecular structure of 18F-PBR is shown in Figure 1, and detailed chemical information of PBR111 can be found in pubchem with the following link: https://pubchem.ncbi.nlm.nih.gov/substance/56431711#section=Top
Van Camp et al. compared 11C-PK11195 and 18F-PBR111 PET imaging in rats with acute neuroinflammation induced by AMPA excitotoxin [6]. PET imaging showed an increased accumulation of 18F-PBR111 in the lesion as compared to the contralateral side as early as 6 min after injection. In addition, the PBR111 probe had much higher affinity and selectivity compared to PK11195 in terms of PBR binding. Since then, 18F-PBR111 has been used in several animal models of neurological diseases, including epilepsy [7, 8], the experimental autoimmune encephalomyelitis model of multiple sclerosis [9], a schizophrenia model [10], etc. The probe has been also used in several clinical trials with healthy subjects or patients [11-13], although 18F-PBR111 is currently not FDA-approved yet.
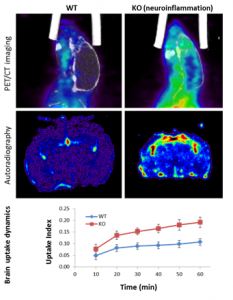
The 18F-PBR111 probe is now available through the BRIC Cyclotron and Radiochemistry facility for preclinical imaging. The SAI facility has conducted PET imaging studies with the 18F-PBR111 on the mouse neuroinflammation models. Both dynamic and static acquisition can be performed to track the uptake of PRB111 in mouse brain (Dynamic acquisition), and uptake level at certain time post injection (Static acquisition). Figure 2 shows the PET images and autoradiography in a study where 18F-PBR111 was injected into either wild type or knockout (KO) mouse pup of neuroinflammation model (PI: Mark Zylka, PhD). Significant increase was observed in the inflammatory brain of a mouse pup. We have also conducted the 18F-PBR111 PET/CT imaging and MRI on a cuprizone-treated mouse multiple sclerosis model, as shown in Figure-3 (PI: Valerie Jewells, DO).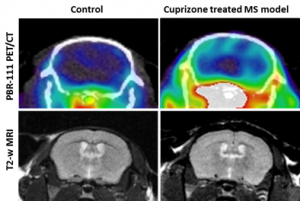
Figure 3. [18]F-PBR111 PET/CT and MRI on control and cuprizone treated multiple sclerosis mouse model.
For more information on the production and availability of the PBR111 probe, please contact Dr. Zibo Li (zibo_li@med.unc.edu), the Director of the Cyclotron and Radiochemistry program. For any imaging and animal protocol related information, please contact Dr. Hong Yuan (yuanh@med.unc.edu) or SAIF at bricsai@med.unc.edu.