
An interdisciplinary team of UNC-Chapel Hill researchers from computational medicine, genetics, biostatistics, and surgery investigated how cell cycle flexibility allows tumor cells to escape the effect of anti-cancer drugs that target cell division. UNC Lineberger members Jeremy Purvis, PhD, professor of genetics, and Phillip Spanheimer, MD, assistant professor of surgery, led this study.
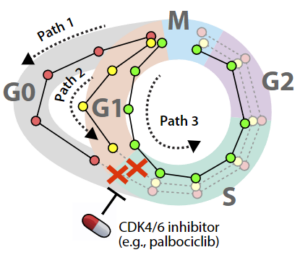
Drugs that inhibit tumor cell cycle, such as palbociclib, have helped treat patients with the breast cancer subtype estrogen receptor–positive, human epidermal growth factor 2 receptor–negative (ER+/HER2−). Although these drugs often improve patient outcomes, a small percentage of tumor cells survive and divide in the presence of palbociclib — a phenomenon known as fractional resistance. It is critical to understand the cellular mechanisms underlying fractional resistance because the precise percentage of resistant cells in patient tissue is a strong predictor of clinical outcomes.
In a study published in the Proceedings of the National Academy of Sciences, a team of UNC-Chapel Hill researchers showed that fractional resistance arises from cell-to-cell differences in core cell cycle regulator proteins that allow a subset of cells to escape palbociclib therapy. The team combined multiplex, single-cell imaging to identify fractionally resistant cells in both cultured and primary breast tumor samples resected from patients. Resistant cells showed premature accumulation of multiple cell cycle regulator proteins as well as enhanced sensitivity to pharmacological inhibition of cyclin-dependent kinase 2 activity, which is another cell division program and another potential drug target.
Using computational approaches to trace the path of tumor cells through the cell cycle, the researchers showed how flexibility, or plasticity, among cell cycle regulators gives rise to alternate cell cycle “paths” that allow individual tumor cells to escape palbociclib treatment.
Understanding drivers of cell cycle plasticity and how to eliminate resistant cell cycle paths, could lead to improved cancer therapies targeting fractionally resistant cells. And Improving such therapies would likely improve patient outcomes.
Co-corresponding authors of the paper are Phillip Spanheimer, MD, assistant professor of surgery, and Jeremy Purvis, PhD, professor of genetics, both at the UNC School of Medicine members of the UNC Lineberger Comprehensive Cancer Center.
This article originally appeared in the UNC Health and UNC School of Medicine Newsroom HERE.
