Specialty Areas:
Normal Physiology of Airway Surface Liquids (ASL); ASL System Failure in CF and COPD
Research Focus:
Dr. Boucher’s lab has focused on the normal physiology of airway surface liquids (ASL) and how this system fails in airways diseases, e.g., cystic fibrosis and COPD. The lab pursues a variety of investigations into the functions of airway epithelia in health and disease. In general terms, the lab focuses on five interrelated areas of research:
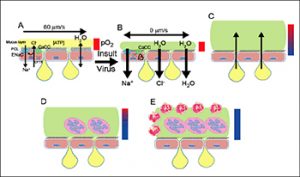
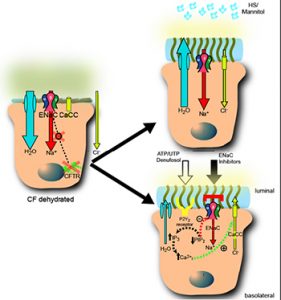
- Airway Surface Liquid (ASL) physiology in health and how it fails in major airways diseases, e.g. the “dehydration hypothesis as relates to CF” (Figure 1).
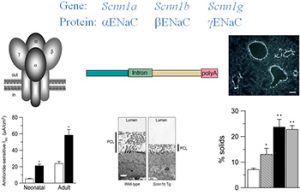
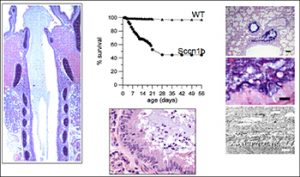
- Airway epithelial Na+ transport, including studies of normal regulation of airway epithelial Na+ transport, and role of Na+ transport in airways disease, including mouse models over-expressing subunits of the epithelial Na+ channel (ENaC) (Figures 2, 3).
- Extracellular nucleotides and their role in airways homeostasis, including the role of P2 receptors in airway epithelial function, extracellular nucleotidases; and measuring the rates of metabolism of nucleotides and nucleotide release from cells.
- Gene therapy for CF lung disease, particularly focusing on investigations of barriers to gene transfer, and improved vector targeting.
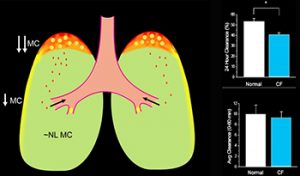
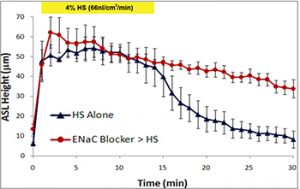
- Clinical studies: a current area of interest focuses on Na+ channel blockers and hypertonic saline (Figures 4-6).
Selected Bibliography:
- Asakura T, Okuda K, Chen G, Dang H, Kato T, Mikami Y, Schworer SA, Gilmore RC, Radicioni G, Hawkins P, Barbosa Cardenas SM, Saito M, Cawley AM, De la Cruz G, Chua M, Alexis NE, Masugi Y, Noone PG, Ribeiro CMP, Kesimer M, Olivier KN, Hasegawa N, Randell SH, O’Neal WK, Boucher RC. Proximal and distal bronchioles contribute to the pathogenesis of non-cystic fibrosis bronchiectasis (NCFB). Am J Respir Crit Care Med. 2024 Feb 15;209(4):374-389. doi: 10.1164/rccm.202306-1093OC. PMID: 38016030. PMCID: PMC10878387.
- Opron K, Begley LA, Erb-Downward JR, Li G, Alexis NE, Barjaktarevic I, Barr RG, Bleecker ER, Boucher R, Bowler RP, Christenson SA, Comellas AP, Criner G, Cooper CB, Couper D, Galban CJ, Han MK, Hastie A, Hatt C, Hoffman EA, Kaner RJ, Kesimer M, Krishnan JA, LaFon DC, Martinez FJ, Ortega VE, Peters SP, Paine Iii R, Putcha N, Woodruff PG, Huffnagle GB, Kozik AJ, Curtis JL, Huang YJ; SPIROMICS Investigators. Loss of airway phylogenetic diversity is associated with clinical and pathobiological markers of disease development in COPD. Am J Respir Crit Care Med. 2024 Jan 23. doi: 10.1164/rccm.202303-0489OC. Epub ahead of print. PMID: 38261629.
- Einarsson GG, Sherrard LJ, Hatch JE, Zorn B, Johnston E, McGettigan C, O’Neill K, Gilpin DF, Downey DG, Murray M, Lavelle G, McElvaney G, Wolfgang MC, Boucher R, Muhlebach MS, Bradbury I, Elborn JS, Tunney MM. Longitudinal changes in the cystic fibrosis airway microbiota with time and treatment. J Cyst Fibros. 2023 Dec 28:S1569-1993(23)01681-8. doi: 10.1016/j.jcf.2023.11.010. Epub ahead of print. PMID: 38158284.
- Nakakubo S*, Kishida N*, Okuda K*, Kamada K, Iwama M, Suzuki M, Yokota I, Ito YM, Nasuhara Y, Boucher RC, Konno S. Associations of COVID-19 symptoms with omicron subvariants BA.2 and BA.5, host status, and clinical outcomes in Japan: A registry-based observational study. Lancet Infect Dis. 2023 Nov;23(11):1244-1256. doi: 10.1016/S1473-3099(23)00271-2. PMID: 37399831. PMCID: PMC10615696. *These authors equally contributed to the work.
- Wang S, Niroula S, Hoffman A, Khorrami M, Khorrami M, Yuan F, Gasser GN, Choi S, Liu B, Li J, Metersky ML, Vincent M, Crum CP, Boucher RC, Karmouty-Quintana H, Huang HJ, Sheshadri A, Dickey BF, Parekh KR, Engelhardt JF, McKeon FD, Xian W. Inflammatory activity of epithelial stem cell variants from cystic fibrosis lung is not resolved by CFTR modulators. Am J Respir Crit Care Med. 2023 Nov 1;208(9):930-943. doi: 10.1164/rccm.202305-0818OC. PMID: 37695863. PMCID: PMC10870857.
- Leach T, Gandhi U, Reeves KD, Stumpf K, Okuda K, Marini FC, Walker SJ, Boucher R, Chan J, Cox LA, Atala A, Murphy SV. Development of a novel air-liquid interface airway tissue equivalent model for in vitro respiratory modeling studies. Sci Rep. 2023 Jun 22;13(1):10137. doi: 10.1038/s41598-023-36863-1. PMID: 37349353; PMCID: PMC10287689.
- Mikami Y, Grubb BR, Rogers TD, Dang H, Asakura T, Kota P, Gilmore RC, Okuda K, Morton LC, Sun L, Chen G, Wykoff JA, Ehre C, Vilar J, van Heusden C, Livraghi-Butrico A, Gentzsch M, Button B, Stutts MJ, Randell SH, O’Neal WK, Boucher RC. Chronic airway epithelial hypoxia exacerbates injury in muco-obstructive lung disease through mucus hyperconcentration. Sci Transl Med. 2023 Jun 7;15(699):eabo7728. doi: 10.1126/scitranslmed.abo7728. PMID: 37285404. PMCID: PMC10664029.
- DiLillo KM, Norman KC, Freeman CM, Christenson SA, Alexis NE, Anderson WH, Barjaktarevic IZ, Barr RG, Comellas AP, Bleecker ER, Boucher RC, Couper DJ, Criner GJ, Doerschuk CM, Wells JM, Han MK, Hoffman EA, Hansel NN, Hastie AT, Kaner RJ, Krishnan JA, Labaki WW, Martinez FJ, Meyers DA, O’Neal WK, Ortega VE, Paine R 3rd, Peters SP, Woodruff PG, Cooper CB, Bowler RP, Curtis JL, Arnold KB; SPIROMICS investigators. A blood and bronchoalveolar lavage protein signature of rapid FEV1 decline in smoking-associated COPD. Sci Rep. 2023 May 22;13(1):8228. doi: 10.1038/s41598-023-32216-0. PMID: 37217548; PMCID: PMC10203309.
- Pearson J, Wessler T, Chen A, Boucher RC, Freeman R, Lai SK, Pickles R, Gregory Forest M. Modeling identifies variability in SARS-CoV-2 uptake and eclipse phase by infected cells as principal drivers of extreme variability in nasal viral load in the 48 hours post infection. J Theor Biol. 2023 May 21;565:111470. doi: 10.1016/j.jtbi.2023.111470. PMID: 36965846; PMCID: PMC10033495.
- Barnett KC, Xie Y, Asakura T, Song D, Liang K, Taft-Benz SA, Guo H, Yang S, Okuda K, Gilmore RC, Loome JF, Oguin III TH, Sempowski GD, Randell SH, Heise MT, Leo Lei Y†, Boucher RC†, Ting JPY†. An epithelial-immune circuit amplifies inflammasome and IL-6 responses to SARS-CoV-2. Cell Host Microbe. 2023 Feb 8;31(2):243-259.e6. doi: 10.1016/j.chom.2022.12.005. PMID: 36563691. PMCID: PMC9731922. †These authors equally supervised this work.
- Esther CR Jr, O’Neal WK, Alexis NE, Koch AL, Cooper CB, Barjaktarevic I, Raffield LM, Bowler RP, Comellas AP, Peters SP, Hastie AT, Curtis JL, Ronish B, Ortega VE, Wells JM, Halper-Stromberg E, Rennard SI, Boucher RC. Prolonged, physiologically relevant nicotine concentrations in the airways of smokers. Am J Physiol Lung Cell Mol Physiol. 2023 Jan 1;324(1):L32-L37. doi: 10.1152/ajplung.00038.2022. PMID: 36342131. PMCID: PMC9829458.
- Kato T, Asakura T, Edwards CE, Dang H, Mikami Y, Okuda K, Chen G, Sun L, Gilmore RC, Hawkins P, De la Cruz G, Cooley MR, Bailey AB, Hewitt SM, Chertow DS, Borczuk AC, Salvatore S, Martinez FJ, Thorne LB, Askin FB, Ehre C, Randell SH, O’Neal WK, Baric RS, Boucher RC; NIH COVID-19 Autopsy Consortium. Prevalence and mechanisms of mucus accumulation in COVID-19 lung disease. Am J Respir Crit Care Med. 2022 Dec 1;206(11):1336-1352. doi: 10.1164/rccm.202111-2606OC. PMID: 35816430. PMCID: PMC9746856.
- Hsu AP, Korzeniowska A, Aguilar CC, Gu J, Karlins E, Oler AJ, Chen G, Reynoso GV, Davis J, Chaput A, Peng T, Sun L, Lack JB, Bays DJ, Stewart ER, Waldman SE, Powell DA, Donovan FM, Desai JV, Pouladi N, Long Priel DA, Yamanaka D, Rosenzweig SD, Niemela JE, Stoddard J, Freeman AF, Zerbe CS, Kuhns DB, Lussier YA, Olivier KN, Boucher RC, Hickman HD, Frelinger J, Fierer J, Shubitz LF, Leto TL, Thompson III GR, Galgiani JN, Lionakis MS, Holland SM. Immunogenetics associated with severe coccidioidomycosis. JCI Insight. 2022 Nov 22;7(22):e159491. doi: 10.1172/jci.insight.159491. PMID: 36166305. PMCID: PMC9746810.
- Markovetz MR, Garbarine IC, Morrison CB, Kissner WJ, Seim I, Forest MG, Papanikolas MJ, Freeman R, Ceppe A, Ghio A, Alexis NE, Stick SM, Ehre C, Boucher RC, Esther CR, Muhlebach MS, Hill DB. Mucus and mucus flake composition and abundance reflect inflammatory and infection status in cystic fibrosis. J Cyst Fibros. 2022 Nov;21(6):959-966. doi: 10.1016/j.jcf.2022.04.008. PMID: 35437233.
- Caetano AJ; Human Cell Atlas Oral and Craniofacial Bionetwork: Sharpe P, Volponi AA, Yianni V, Bush M, McKay LK, Wallet S, Shazib MA, Kimple A, Easter Q, Weaver T, Sequeira I, Kumar R, Pereira D, Macken J, Fortune F, Efremova M, Teichmann S, Kretzschmar K, Todres E, Boffelli D, Hagood JS, Klein O, Boucher R, Lwin S, Pranzatelli T, Beachy P, Lu WJ, Miller Zmora I, Moutsopoulos N, Williams DW, Pringle S, Krivanek J, Warner BM, Perez P, Opasawatchai A, Matuck B, Freire M, Tata PR, Conde CD, Haniffa M, Pisco AO, Momen-Heravi F, Kapila Y, Gulati A; Sequeira I, Byrd KM. A roadmap for the Human Oral and Craniofacial Cell Atlas. J Dent Res. 2022 Oct;101(11):1274-1288. doi: 10.1177/00220345221110768. PMID: 36154725. PMCID: PMC9516614.
- Hill DB, Button B, Rubinstein M, Boucher RC. Physiology and pathophysiology of human airway mucus. Physiol Rev. 2022 Oct 1;102(4):1757-1836. doi: 10.1152/physrev.00004.2021. PMID: 35001665. PMCID: PMC9665957.
- Dinnon KH§, Leist SR§, Okuda K§, Dang H§, Fritch EJ§, Gully KL, De la Cruz G, Evangelista MD, Asakura T, Gilmore RC, Hawkins P, Nakano S, West A, Schäfer A, Gralinski LE, Everman JL, Sajuthi SP, Zweigart MR, Dong S, McBride J, Cooley MR, Hines JB, Love MK, Groshong SD, VanSchoiack A, Phelan SJ, Liang Y, Hether T, Leon M, Zumwalt RE, Barton LM, Duval EJ, Mukhopadhyay S, Stroberg E, Borczuk A, Thorne LB, Sakthivel MK, Lee YZ, Hagood JS, Mock JR, Seibold MA, O’Neal WK, Montgomery SA, Boucher RC*, Baric RS*. SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci Transl Med. 2022 Sep 28;14(664):eabo5070. doi: 10.1126/scitranslmed.abo5070. PMID: 35857635. PMCID: PMC9273046. §These authors contributed equally. *Joint senior authorship.
- Esther CR Jr, Kimura KS, Mikami Y, Edwards CE, Das SR, Freeman MH, Strickland BA, Brown HM, Wessinger BC, Gupta VC, Von Wahlde K, Sheng Q, Huang LC, Bacon DR, Kimple AJ, Ceppe AS, Kato T, Pickles RJ, Randell SH, Baric RS, Turner JH*, Boucher RC*. Pharmacokinetic-based failure of a detergent virucidal for SARS-COV-2 nasal infections: A preclinical study and randomized controlled trial. Int Forum Allergy Rhinol. 2022 Sep;12(9):1137-1147. doi: 10.1002/alr.22975. PMID: 35040594. PMCID: PMC9011886. *Joint senior authorship.
- Schäfer A, Leist SR, Gralinski LE, Martinez DR, Winkler ES, Okuda K, Hawkins PE, Gully KL, Graham RL, Scobey DT, Bell TA, Hock P, Shaw GD, Loome JF, Madden EA, Anderson E, Baxter VK, Taft-Benz SA, Zweigart MR, May SR, Dong S, Clark M, Miller DR, Lynch RM, Heise MT, Tisch R, Boucher RC, Pardo Manuel de Villena F, Montgomery SA, Diamond MS, Ferris MT, Baric RS. A Multitrait Locus Regulates Sarbecovirus Pathogenesis. mBio. 2022 Aug 30;13(4):e0145422. doi: 10.1128/mbio.01454-22. PMID: 35862771. PMCID: PMC9426612.
- Batson B, Zorn B, Radicioni G, Livengood S, Kumagai T, Dang H, Ceppe A, Clapp P, Tunney M, Elborn S, McElvaney G, Muhlebach M, Boucher RC, Tiemeyer M, Wolfgang M, Kesimer M. Cystic fibrosis airway mucus hyperconcentration produces a vicious cycle of mucin, pathogen, and inflammatory interactions that promote disease persistence. Am J Respir Cell Mol Biol. 2022 Aug;67(2):253-265. doi: 10.1165/rcmb.2021-0359OC. PMID: 35486871. PMCID: PMC9348562.
- Biering SB, Sarnik SA, Wang E, Zengel JR, Leist SR, Schäfer A, Sathyan V, Hawkins P, Okuda K, Tau C, Jangid AR, Duffy CV, Wei J, Gilmore RC, Alfajaro MM, Strine MS, Nguyenla X, Van Dis E, Catamura C, Yamashiro LH, Belk JA, Begeman A, Stark JC, Shon DJ, Fox DM, Ezzatpour S, Huang E, Olegario N, Rustagi A, Volmer AS, Livraghi-Butrico A, Wehri E, Behringer RR, Cheon DJ, Schaletzky J, Aguilar HC, Puschnik AS, Button B, Pinsky BA, Blish CA, Baric RS, O’Neal WK, Bertozzi CR, Wilen CB, Boucher RC, Carette JE, Stanley SA, Harris E, Konermann S, Hsu PD. Genome-wide bidirectional CRISPR screens identify mucins as host factors modulating SARS-CoV-2 infection. Nat Genet. 2022 Aug;54(8):1078-1089. doi: 10.1038/s41588-022-01131-x. PMID: 35879412. PMCID: PMC9355872.
- Kim N, Kwak G, Rodriguez J, Livraghi-Butrico A, Zuo X, Simon V, Han E, Shenoy SK, Pandey N, Mazur M, Birket SE, Kim A, Rowe SM, Boucher R, Hanes J, Suk JS. Inhaled gene therapy of preclinical muco-obstructive lung diseases by nanoparticles capable of breaching the airway mucus barrier. Thorax. 2022 Aug;77(8):812-820. doi: 10.1136/thoraxjnl-2020-215185. PMID: 34697091. PMCID: PMC9129924.
- Ward JD, Cornaby C, Kato T, Gilmore RC, Bunch D, Miller MB, Boucher RC, Schmitz JL, Askin FA, Scanga LR. The clinical impact of maternal COVID-19 on mothers, their infants, and placentas with an analysis of vertical transfer of maternal SARS-CoV-2-specific IgG antibodies. Placenta. 2022 Jun 1;123:12-23. doi: 10.1016/j.placenta.2022.04.006. PMID: 35512490; PMCID: PMC9057562.
- Chen A, Wessler T, Daftari K, Hinton K, Boucher RC, Pickles R, Freeman R, Lai SK, Forest MG. Modeling insights into SARS-CoV-2 respiratory tract infections prior to immune protection. Biophys J. 2022 May 3;121(9):1619-1631. doi: 10.1016/j.bpj.2022.04.003. PMID: 35378080; PMCID: PMC8975607.
- Esther CR Jr, O’Neal WK, Anderson WH, Kesimer M, Ceppe A, Doerschuk CM, Alexis NE, Hastie AT, Barr RG, Bowler RP, Wells JM, Oelsner EC, Comellas AP, Tesfaigzi Y, Kim V, Paulin LM, Cooper CB, Han MK, Huang YJ, Labaki WW, Curtis JL, Boucher RC; SPIROMICS. Identification of sputum biomarkers predictive of pulmonary exacerbations in chronic obstructive pulmonary disease. Chest. 2022 May;161(5):1239-1249. doi: 10.1016/j.chest.2021.10.049. PMID: 34801592. PMCID: PMC9131049.
- Morrison CB, Edwards CE, Shaffer KM, Araba KC, Wykoff JA, Williams DR, Asakura T, Dang H, Morton LC, Gilmore RC, O’Neal WK, Boucher RC, Baric RS, Ehre C. SARS-CoV-2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL-13. Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2119680119. doi: 10.1073/pnas.2119680119. PMID: 35353667. PMCID: PMC9169748.
- Singanayagam A, Footitt J, Marczynski M, Radicioni G, Cross MT, Finney LJ, Trujillo-Torralbo MB, Calderazzo MA, Zhu J, Aniscenko J, Clarke TB, Molyneaux PL, Bartlett NW, Moffatt MF, Cookson WO, Wedzicha JA, Evans CM, Boucher RC, Kesimer M, Lieleg O, Mallia P, Johnston SL. Airway mucins promote immunopathology in virus-exacerbated chronic obstructive pulmonary disease. J Clin Invest. 2022 Apr 15;132(8):e120901. doi: 10.1172/JCI120901. PMID: 35239513. PMCID: PMC9012283.
- Kadur Lakshminarasimha Murthy P, Sontake V, Tata A, Kobayashi Y, Macadlo L, Okuda K, Conchola AS, Nakano S, Gregory S, Miller LA, Spence JR, Engelhardt JF, Boucher RC, Rock JR, Randell SH, Tata PR. Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature. 2022 Apr;604(7904):111-119. doi: 10.1038/s41586-022-04541-3. PMID: 35355018. PMCID: PMC9169066.
- Kato T, Radicioni G, Papanikolas MJ, Stoychev GV, Markovetz MR, Aoki K, Porterfield M, Okuda K, Barbosa Cardenas SM, Gilmore RC, Morrison CB, Ehre C, Burns KA, White KK, Brennan TA, Goodell HP, Thacker H, Loznev HT, Forsberg LJ, Nagase T, Rubinstein M, Randell SH, Tiemeyer M, Hill DB, Kesimer M, O’Neal WK, Ballard ST, Freeman R, Button B, Boucher RC. Mucus concentration-dependent biophysical abnormalities unify submucosal gland and superficial airway dysfunction in cystic fibrosis. Sci Adv. 2022 Apr;8(13):eabm9718. doi: 10.1126/sciadv.abm9718. PMID: 35363522.
- McElvaney OF, Asakura T, Meinig SL, Torres-Castillo JL, Hagan RS, Gabillard C, Murphy MP, Thorne LB, Borczuk A, Reeves EP, Zumwalt RE, Mikami Y, Carroll TP, Okuda K, Hogan G, McElvaney OJ, Clarke J, McEvoy NL, Mallon PW, McCarthy C, Curley G, Wolfgang MC, Boucher RC, McElvaney NG. Protease-anti-protease compartmentalization in SARS-CoV-2 ARDS: Therapeutic implications. EBioMedicine. 2022 Mar;77:103894. doi: 10.1016/j.ebiom.2022.103894. PMID: 35217407; PMCID: PMC8861575.
- Shi Y, Zeida A, Edwards CE, Mallory ML, Sastre S, Machado MR, Pickles RJ, Fu L, Liu K, Yang J, Baric RS, Boucher RC, Radi R, Carroll KS. Thiol-based chemical probes exhibit antiviral activity against SARS-CoV-2 via allosteric disulfide disruption in the spike glycoprotein. Proc Natl Acad Sci U S A. 2022 Feb 8;119(6):e2120419119. doi: 10.1073/pnas.2120419119. PMID: 35074895. PMCID: PMC8833197.
- Ford AG, Cao XZ, Papanikolas MJ, Kato T, Boucher RC, Markovetz MR, Hill DB, Freeman R, Forest MG. Molecular Dynamics Simulations to Explore the Structure and Rheological Properties of Normal and Hyperconcentrated Airway Mucus. Stud Appl Math. 2021 Nov;147(4):1369-1387. doi: 10.1111/sapm.12433. PMID: 35221375; PMCID: PMC8871504.
- Radicioni G, Ceppe A, Ford AA, Alexis NE, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez FJ, Ozkan E, Paine R 3rd, Woodruff PG, O’Neal WK, Boucher RC, Kesimer M. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2021 Nov;9(11):1241-1254. doi: 10.1016/S2213-2600(21)00079-5. PMID: 34058148. PMCID: PMC8570975.
- Bennett WD, Burbank A, Almond M, Wu J, Ceppe A, Hernandez M, Boucher RC, Peden DB. Acute and durable effect of inhaled hypertonic saline on mucociliary clearance in adult asthma. ERJ Open Res. 2021 Jun 7;7(2):00062-2021. doi: 10.1183/23120541.00062-2021. PMID: 34109248; PMCID: PMC8184161.
- Kato T, Mikami Y, Sun L, Rogers TD, Grubb BR, Morrison CB, Ehre C, Sears PR, Ostrowski LE, Randell SH, Boucher RC. Reuse of Cell Culture Inserts for in vitro Human Primary Airway Epithelial Cell Studies. Am J Respir Cell Mol Biol. 2021 Jun;64(6):760-764. doi: 10.1165/rcmb.2021-0033LE. PMID: 33788673. PMCID: PMC8456889.
- Okuda K, Dang H, Kobayashi Y, Carraro G, Nakano S, Chen G, Kato T, Asakura T, Gilmore RC, Morton LC, Lee RE, Mascenik T, Yin WN, Barbosa Cardenas SM, O’Neal YK, Minnick CE, Chua M, Quinney NL, Gentzsch M, Anderson CW, Ghio A, Matsui H, Nagase T, Ostrowski LE, Grubb BR, Olsen JC, Randell SH, Stripp BR, Tata PR, O’Neal WK, Boucher RC. Secretory Cells Dominate Airway CFTR Expression and Function in Human Airway Superficial Epithelia. Am J Respir Crit Care Med. 2021 May 15;203(10):1275-1289. doi: 10.1164/rccm.202008-3198OC. PMID: 33321047. PMCID: PMC8456462.
- Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, Pelayo E, Maldonado JO, Lafont BA, Jang SI, Nasir N, Padilla RJ, Murrah VA, Maile R, Lovell W, Wallet SM, Bowman NM, Meinig SL, Wolfgang MC, Choudhury SN, Novotny M, Aevermann BD, Scheuermann RH, Cannon G, Anderson CW, Lee RE, Marchesan JT, Bush M, Freire M, Kimple AJ, Herr DL, Rabin J, Grazioli A, Das S, French BN, Pranzatelli T, Chiorini JA, Kleiner DE, Pittaluga S, Hewitt SM, Burbelo PD, Chertow D; NIH COVID-19 Autopsy Consortium; HCA Oral and Craniofacial Biological Network, Frank K, Lee J, Boucher RC, Teichmann SA, Warner BM, Byrd KM. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021 May;27(5):892-903. doi: 10.1038/s41591-021-01296-8. PMID: 33767405. PMCID: PMC8240394.
- Lazarowski ER, Boucher RC. Purinergic receptors in airway hydration. Biochem Pharmacol. 2021 May;187:114387. doi: 10.1016/j.bcp.2020.114387. PMID: 33358825. PMCID: PMC8096650.
- Dunican EM, Elicker BM, Henry T, Gierada DS, Schiebler ML, Anderson W, Barjaktarevic I, Barr RG, Bleecker ER, Boucher RC, Bowler RP, Christenson SA, Comellas A, Cooper CB, Couper D, Criner GJ, Dransfield M, Doerschuk CM, Drummond MB, Hansel NN, Han MK, Hastie AT, Hoffman EA, Krishnan JA, Lazarus SC, Martinez FJ, McCulloch CE, O’Neal WK, Ortega VE, Paine R 3rd, Peters S, Schroeder JD, Woodruff PG, Fahy JV. Mucus plugs and emphysema in the pathophysiology of airflow obstruction and hypoxemia in smokers. Am J Respir Crit Care Med. 2021 Apr 15;203(8):957-968. doi: 10.1164/rccm.202006-2248OC. PMID: 33180550. PMCID: PMC8048745.
- Xu J, Livraghi-Butrico A, Hou X, Rajagopalan C, Zhang J, Song J, Jiang H, Wei HG, Wang H, Bouhamdan M, Ruan J, Yang D, Qiu Y, Youming X, Barrett RP, McClellan SA, Mou H, Wu Q, Chen X, Rogers TD, Wilkinson KJ, Gilmore RC, Esther CR Jr, Zaman K, Liang X, Sobolic M, Hazlett L, Zhang K, Frizzell RA, Gentzsch M, O’Neal WK, Grubb BR, Chen YE, Boucher RC, Sun F. Phenotypes of CF rabbits generated by CRISPR/Cas9-mediated disruption of the CFTR gene. JCI Insight. 2021 Jan 11;6(1):e139813. doi: 10.1172/jci.insight.139813. PMID: 33232302. PMCID: PMC7821608.
- Hou YJ, Chiba S, Halfmann P, Ehre C, Kuroda M, Dinnon KH 3rd, Leist SR, Schäfer A, Nakajima N, Takahashi K, Lee RE, Mascenik TM, Graham R, Edwards CE, Tse LV, Okuda K, Markmann AJ, Bartelt L, de Silva A, Margolis DM, Boucher RC, Randell SH, Suzuki T, Gralinski LE, Kawaoka Y, Baric RS. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020 Dec 18;370(6523):1464-1468. doi: 10.1126/science.abe8499. PMID: 33184236. PMCID: PMC7775736.
- Leist SR, Dinnon KH 3rd, Schäfer A, Tse LV, Okuda K, Hou YJ, West A, Edwards CE, Sanders W, Fritch EJ, Gully KL, Scobey T, Brown AJ, Sheahan TP, Moorman NJ, Boucher RC, Gralinski LE, Montgomery SA, Baric RS. A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell. 2020 Nov 12;183(4):1070-1085.e12. doi: 10.1016/j.cell.2020.09.050. PMID: 33031744; PMCID: PMC7510428.
- Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon III KH, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Fulcher L, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O’Neal WK, Randell SH, Boucher RC*, Baric RS*. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020 Jul 23;182(2):429-446.e14. doi: 10.1016/j.cell.2020.05.042. PMID: 32526206; PMCID: PMC7250779. *Co-senior authors.
- Ramsey KA, Chen ACH, Radicioni G, Lourie R, Martin M, Broomfield A, Sheng YH, Hasnain SZ, Radford-Smith G, Simms L, Burr L, Thornton DJ, Bowler SD, Livengood S, Ceppe A, Knowles MR, Donaldson SH, Hill DB, Ehre C, Button B, Alexis NE, Kesimer M, McGuckin MA*, Boucher RC*. Airway mucus hyperconcentration in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2020 Mar 15;201(6):661-670. doi: 10.1164/rccm.201906-1219OC. PMID: 31765597. PMCID: PMC7068838. *Co-senior authors.
- Chen G, Sun L, Kato T, Okuda K, Martino MB, Abzhanova A, Lin JM, Gilmore RC, Batson BD, Volmer AS, Dang H, Deng Y, Randell SH, Button B, Livraghi-Butrico A, Kesimer M, Ribeiro CMP, O’Neal WK, Boucher RC. IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J Clin Invest. 2019 Oct 1;129(10):4433-4450. doi: 10.1172/JCI125669. PMID: 31524632.
- Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, Martino MB, Dang H, Abzhanova A, Lin JM, Hull-Ryde EA, Volmer AS, Randell SH, Livraghi-Butrico A, Deng Y, Scherer PE, Stripp BR, O’Neal WK, Boucher RC. XBP1S regulates MUC5B in a promoter variant-dependent pathway in IPF airway epithelia. Am J Respir Crit Care Med. 2019 Jul 15;200(2):220-234. doi: 10.1164/rccm.201810-1972OC. PMID: 30973754. PMCID: PMC6635783.
- Boucher RC. Muco-obstructive lung diseases. N Engl J Med. 2019 May 16;380(20):1941-1953. doi: 10.1056/NEJMra1813799. PMID: 31091375.
- Esther CR Jr, Muhlebach MS, Ehre C, Hill DB, Wolfgang MC, Kesimer M, Ramsey KA, Markovetz MR, Garbarine IC, Forest MG, Seim I, Zorn B, Morrison CB, Delion MF, Thelin WR, Villalon D, Sabeter JR, Turkovic L, Ranganathan S, Stick SM, Boucher RC, on behalf of AREST CF. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci Transl Med. 2019 Apr 3;11(486). doi: 10.1126/scitranslmed.aav3488. PMID: 30944166. PMCID: PMC6566903.
- Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, Radicioni G, Kesimer M, Chua M, Livraghi-Butrico A, Ehre C, Doerschuk CM, Randell SH, Matsui H, Nagase T, O’Neal WK, Boucher RC. Localization of secretory mucins MUC5AC and MUC5B in normal human airways. Am J Respir Crit Care Med. 2019 Mar 15;199(6):715-727. doi: 10.1164/rccm.201804-0734OC. PMID: 30352166. PMCID: PMC6423099.
- Button B, Goodell HP, Atieh E, Chen YC, Williams R, Shenoy S, Lackey E, Shenkute N, Cai L, Dennis R, Boucher RC, Rubinstein M. Roles of mucus adhesion and cohesion in cough clearance. Proc Natl Acad Sci U S A. 2018 Dec 4;115(49):12501-12506. doi: 10.1073/pnas.1811787115. PMID: 30420506. PMCID: PMC6298066.
- Mall M, Danahay H, Boucher RC. Emerging concepts and therapies for mucoobstructive lung disease. Ann Am Thorac Soc. 2018 Nov;15(Supplement_3):S216-S226. doi: 10.1513/AnnalsATS.201806-368AW. PMID: 30431343. PMCID: PMC6322026.
- Chen G, Volmer AS, Wilkinson KJ, Deng Y, Jones LC, Yu D, Bustamante-Marin XM, Burns KA, Grubb BR, O’Neal WK, Livraghi-Butrico A, Boucher RC. Role of Spdef in the regulation of Muc5b expression in the airways of naïve and muco-obstructed mice. Am J Respir Cell Mol Biol. 2018 Sep;59(3):383-396. doi: 10.1165/rcmb.2017-0127OC. PMID: 29579396. PMCID: PMC6189647.
- Abdullah LH, Coakley R, Webster MJ, Zhu Y, Tarran R, Radicioni G, Kesimer M, Boucher RC, Davis CW, Ribeiro CMP. Mucin production and hydration responses to mucopurulent materials in normal vs. CF airway epithelia. Am J Respir Crit Care Med. 2018 Feb 15;197(4):481-491. doi: 10.1164/rccm.201706-1139OC. PMID: 29099608. PMCID: PMC5821906.
- Livraghi-Butrico A, Wilkinson KJ, Volmer AS, Gilmore RC, Rogers TD, Caldwell RA, Burns KA, Esther CR Jr, Mall MA, Boucher RC, O’Neal WK, Grubb BR. Lung disease phenotypes caused by overexpression of combinations of α-, β-, and γ-subunits of the epithelial sodium channel in mouse airways. Am J Physiol Lung Cell Mol Physiol.2018 Feb 1;314(2):L318-L331. doi: 10.1152/ajplung.00382.2017. PMID: 29074490. PMCID: PMC5866504.
- Yu D, Saini Y, Chen G, Ghio AJ, Dang H, Burns KA, Wang Y, Davis RM, Randell SH, Esther Jr CR, Paulsen F, Boucher RC. Loss of βENaC function in meibomian glands produces pseudohypoaldosteronism 1-like ocular disease in mice. Am J Pathol. 2018 Jan;188(1):95-110.doi: 10.1016/j.ajpath.2017.09.016. PMID: 29107074. PMCID: PMC5745530.
- Donoghue LJ*, Livraghi-Butrico A*, McFadden KM, Thomas JM, Chen G, Grubb BR, O’Neal WK, Boucher RC, Kelada SNP. Identification of trans protein QTL for secreted airway mucins in mice and a causal role for Bpifb1. Genetics. 2017 Oct;207(2):801-812. doi: 10.1534/genetics.117.300211. PMID: 28851744. PMCID: PMC5629340.
- Adams DC, Pahlevaninezhad H, Szabari MV, Cho JL, Hamilos DL, Kesimer M, Boucher RC, Luster AD, Medoff BD, Suter MJ. Automated segmentation and quantification of airway mucus with endobronchial optical coherence tomography. Biomed Opt Express. 2017;8(10):4729-4741. doi: 10.1364/BOE.8.004729. PMID: 29082098. PMCID: PMC5654813.
- Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis WC, Doerschuk CM, Alexis NE, Anderson WH, Henderson AG, Barr G, Bleecker ER, Christenson SA, Cooper CB, Han MLK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez F, Paine R, Woodruff PG, O’Neal WK, Boucher RC. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017 Sep 7;377(10):911-922. doi: 10.1056/NEJMoa1701632. PMID: 28877023. PMCID: PMC5706541.
- Sandefur CI, Boucher RC, Elston TC. Mathematical model reveals role of nucleotide signaling in airway surface liquid homeostasis and its dysregulation in cystic fibrosis. Proc Natl Acad Sci U S A. 2017 Aug 29;114(35): E7272-E7281. doi: 10.1073/pnas.1617383114. PMID: 28808008. PMCID: PMC5584404.
- Woodruff PG, van den Berge M, Boucher RC, Brightling C, Burchard EG, Christenson SA, Han MK, Holtzman MJ, Kraft M, Lynch DA, Martinez FD, Reddel HK, Sin DD, Washko GR, Wenzel SE, Punturieri A, Freemer MM, Wise RA. ATS-NHLBI Asthma COPD Overlap (ACO) workshop report. Am J Respir Crit Care Med. 2017 Aug 1;196(3):375-381. doi: 10.1164/rccm.201705-0973WS. PMID: 28636425. PMCID: PMC5549872.
- Esther CR Jr, Hill DB, Button B, Shi S, Jania CM, Duncan EA, Doerschuk CM, Chen G, Ranganathan S, Stick SM, Boucher RC. The sialic acid to urea ratio as a measure of airway surface hydration. Am J Physiol Lung Cell Mol Physiol. 2017 Mar 1;312(3):L398-404. doi: 10.1152/ajplung.00398.2016. PMID: 28062483. PMCID: PMC5374301.
- Livraghi-Butrico A, Grubb BR, Wilkinson K, Volmer AS, Burns KA, Evans C, O’Neal WK, Boucher RC. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2017 Mar;10(2):395-407. doi: 10.1038/mi.2016.63. PMID: 27435107. PMCID: PMC5250616.
- Zeman KL, Rojas Balcazar J, Fuller F, Donn KH, Boucher RC, Bennett WD, Donaldson SH. A trans-nasal aerosol delivery device for efficient pulmonary deposition. J Aerosol Med Pulm Drug Deliv. 2017 Aug;30(4):223-9. doi: 10.1089/jamp.2016.1333. PMID: 28157412. PMCID: PMC5564034.
- Saini Y, Wilkinson KJ, Terrell KA, Burns KA, Livraghi-Butrico A, Doerschuk CM, O’Neal WK, Boucher RC. Neonatal pulmonary macrophage depletion coupled to defective mucus clearance increases susceptibility to pneumonia and alters pulmonary immune responses. Am J Respir Cell Mol Biol. 2016 Feb;54(2):210-21. doi: 10.1165/rcmb.2014-0111OC. PubMed PMID: 26121027. PubMed Central PMCID: PMC4821038.
- Lubamba BA, Jones LC, O’Neal WK, Boucher RC, Ribeiro CM. X-box binding protein 1 modulates innate immune responses of cystic fibrosis alveolar macrophages. Am J Respir Crit Care Med. 2015 Dec 15;192(12):1449-61. doi: 10.1164/rccm.201504-0657OC. PubMed PMID: 26331676. PubMed Central PMCID: PMC4731720.
- Ghosh A, Boucher RC, Tarran R. Airway hydration and COPD. Cell Mol Life Sci. 2015 Oct;72(19):3637-52. doi: 10.1007/s00018-015-1946-7. PubMed PMID: 26068443; PubMed Central PMCID: PMC4567929.
- Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med. 2015 Jul 15;192(2):182-90. doi: 10.1164/rccm.201412-2230OC. PubMed PMID: 25909230; PubMed Central PMCID: PMC4532825.
