Anil Gehi, MD, Streamlining Triage for AFib Patients to Increase Care Coordination and Prevent Hospitalizations
Dr. Anil Gehi’s research focus is on innovative approaches to atrial fibrillation (AFib), from detailed understanding of patient symptomatology, techniques of ablation, processes of care in AFib management, and outcomes research through complex analyses of big data.
In 2017, Dr. Gehi received a 3-year, $1.7 million grant from the Bristol Myers Squibb Foundation to expand his novel AFib treatment model. Dr. Gehi’s program establishes outpatient AFib clinics staffed by advanced practice providers delivering guideline-directed care. AFib patients presenting to the emergency room are triaged, and appropriate patients are transferred to the new outpatient clinics rather than being hospitalized.
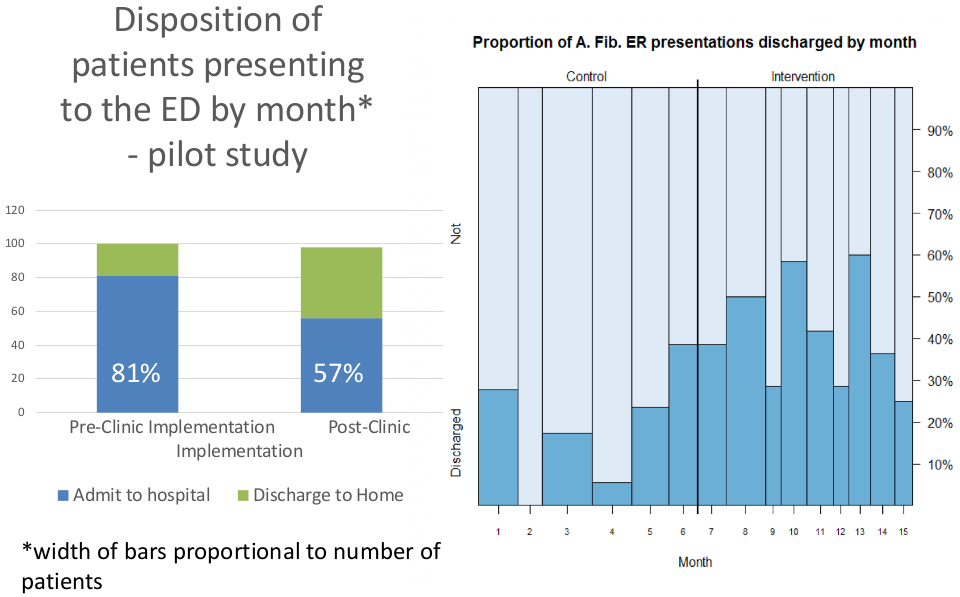
 His initial research in this area was funded through a pilot grant from UNC Health Care’s Center for Innovation and demonstrated promising results: nationwide, 80%-85% of AFib patients presenting to the ER end up hospitalized; Dr. Gehi’s pilot research reduced the percentage of hospitalized patients to 57%. Among other benefits, Dr. Gehi’s model can increase coordination of care for these often complex patients.
His initial research in this area was funded through a pilot grant from UNC Health Care’s Center for Innovation and demonstrated promising results: nationwide, 80%-85% of AFib patients presenting to the ER end up hospitalized; Dr. Gehi’s pilot research reduced the percentage of hospitalized patients to 57%. Among other benefits, Dr. Gehi’s model can increase coordination of care for these often complex patients.
The Bristol Myers Squibb Foundation grant will permit Gehi to expand this model across the State, including to areas where access to specialty care is limited. Dr. Gehi has established partnerships with UNC Hospitals Hillsborough campus, UNC Rex Healthcare, Nash Health Care and Johnston Health.
Additional projects include exploring innovative ways to drive patient engagement in managing chronic conditions such as AFib.
Brian Jensen, MD, Researching How Metabolic Alterations Can Provide Cardioprotection
The Jensen lab recently received an R01 from the NHLBI to investigate the role of alpha-1 adrenergic receptors (α1-ARs) in adaptive regulation of cardiac metabolism in the failing heart.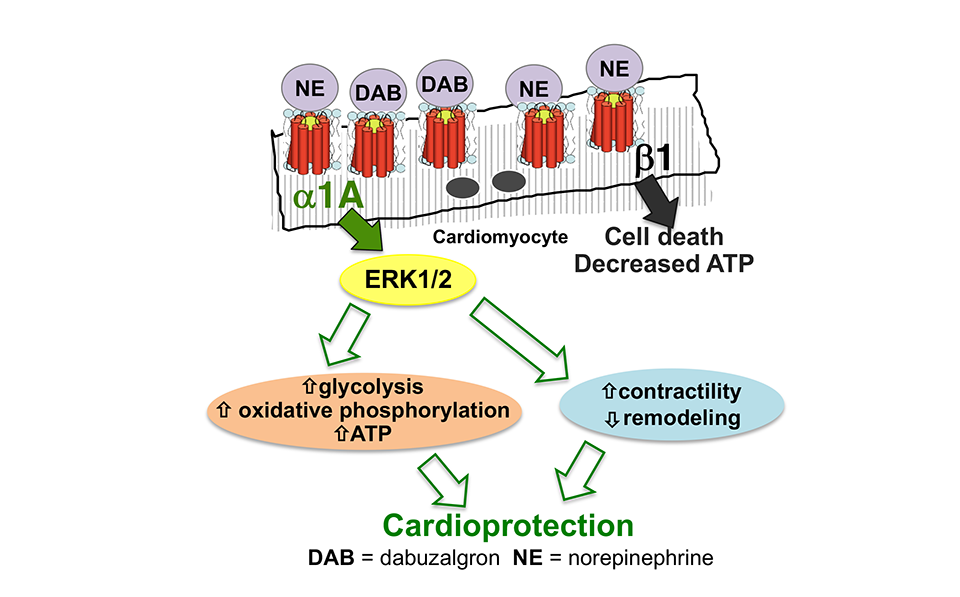
Heart failure (HF) is characterized by markedly elevated levels of catecholamines that bind to adrenergic receptors (ARs) in the heart. The toxic effects of excessive beta (β)-AR stimulation are well described, and drugs that block β-ARs are cornerstones of contemporary HF therapy. Cardiac alpha (α)1-ARs have received less attention, however data from cell and animal studies indicate that they protect against the development of HF. There are two α1-AR subtypes in the heart: α1A, and α1B. The α1B mediates cardiac hypertrophy induced by non-selective α1-AR agonists like phenylephrine. Activation of the α1A protects against cardiomyocyte death and increases contractility in the failing heart, though the mechanisms underlying these adaptive effects are incompletely understood.
The Jensen Lab recently showed that an oral selective α1A agonist drug, dabuzalgron, preserves ATP content and mitochondrial function in mouse HF models. Collectively, Dr. Jensen’s ongoing work challenges the prevailing paradigm that chronic catecholamine surge exerts uniformly deleterious effects in the failing heart.
The Jensen lab also has ongoing projects investigating the mechanisms underlying kinase inhibitor cardiotoxicity and the role of the nuclear receptor ROR-alpha in regulating cardiac hypertrophy and response to injury.
Rick Stouffer, MD, Improving Outcomes for Patients Who Undergo Interventional Procedures
Dr. Rick Stouffer, Chief of Cardiology, Co-Director of the McAllister Heart Institute, and Ernest and Hazel Craige Distinguished Professor of Cardiovascular Medicine, is improving outcomes for patients undergoing heart catheterization and other interventional procedures with three research projects. Results of the first project are highlighted in two recent publications–“Multisite Investigation of Outcomes With Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention” in the Journal of the American College of Cardiology and “Clinical outcomes and sustainability of using CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention,” in Circulation: Genomic and Precision Medicine. These publications are the result of a collaboration of the Division of Cardiology (including Shiv Madan), the School of Pharmacy, and McAllister Heart Institute, and showed that medication selection based on the results of a simple genetic test can improve outcomes for patients undergoing percutaneous coronary intervention (PCI). The genetic test predicts a patient’s response to clopidogrel, a drug commonly prescribed to those undergoing PCI to prevent the formation of blood clots. Research found that patients with a specific genetic alteration have substantially better results with an alternative medication.
A second research area is the use of hemodynamics to predict heart failure and mortality in patients with ST-elevation myocardial infarction (STEMI). Results of this project are highlighted in two recent publications–“Ratio of systolic blood pressure to left ventricular end-diastolic pressure at the time of primary percutaneous coronary intervention predicts in-hospital mortality in patients with ST-elevation myocardial infarction” and “Correlation of Infarct Size With Invasive Hemodynamics in Patients With ST-Elevation Myocardial Infarction” in Catheterization and Cardiovascular Interventions. These studies found that an index of systolic blood pressure to left ventricular end-diastolic pressure correlated with infarct size and mortality in patients with STEMI undergoing primary PCI. This project is a collaboration with various members of the Division of Cardiology including Michael Yeung, Bob Rayson and Melissa Caughey.
Dr. Stouffer has also been a leading investigator on the In-Hospital STEMI Quality Improvement Project, a multiyear study on STEMI that occurs in patients hospitalized for a non-cardiac indication. Historically, research and public health efforts have focused on STEMI occurring outside the hospital setting; STEMI occurring in hospitalized patients has been researched less and these patients have much higher mortality rates. As the founding site for the In-Hospital STEMI Quality Improvement Project, UNC has led efforts to better understand this class of patients and to develop protocols to improve time to diagnosis and treatment. The UNC effort has results in publications in JAMA, Nature Reviews Cardiology and a 2017 publication in JAMA Cardiology which showed that a quality improvement protocol pioneered at UNC improved recognition and treatment times in inpatient STEMI (“A Quality Improvement Program for Recognition and Treatment of Inpatient ST-Segment Elevation Myocardial Infarctions.”
Furthermore, along with Dr. Alan Hinderliter, Dr. Stouffer is an investigator on the RADIANCE-HTN trial, which is looking at the efficacy of renal denervation for treatment of hypertension. Renal denervation is a minimally invasive procedure that interrupts some nerve signals between the brain and the kidneys. RADIANCE-HTN looks at the efficacy of a recently developed ultrasound-based form of renal denervation in two types of patients – those with mildly elevated blood pressure and those with resistant hypertension in which blood pressure is not well-controlled despite the use of three or more medications.
Heart Valve Research at UNC
UNC Valve Team Investigates the Impact of Atrial Fibrillation on Outcomes after Transcatheter Mitral Valve Repair from STS/TVT NCDR Registry
Dr. John Paul Vavalle (Director, Structural Heart Disease)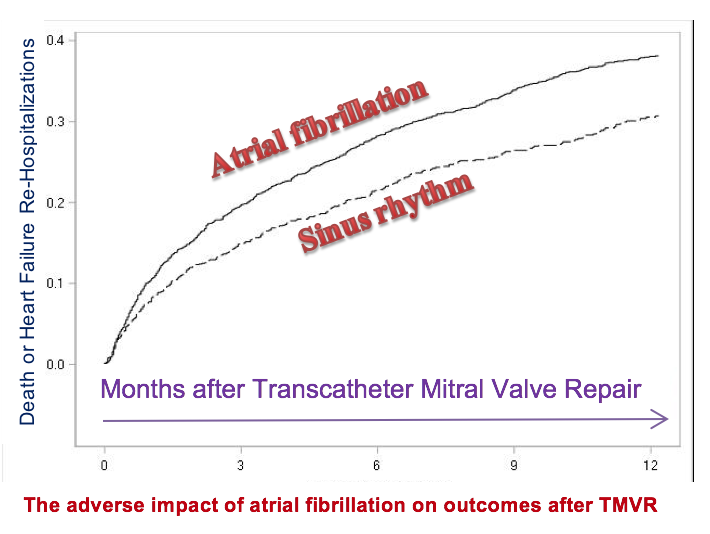 and Dr. Sameer Arora (research fellow with cardiology) will be working with the coordinators of Transcatheter Valve Therapy registry at Duke Clinical Research Institute to investigate the impact of atrial fibrillation on outcomes after transcatheter mitral valve repair (TMVR). TMVR has emerged as a treatment option for severe symptomatic patients with chronic severe primary mitral regurgitation who are not surgical candidates.” Dr. Vavalle has been treating patients with this ground-breaking treatment at UNC since initiating the structural heart disease program in 2014. Atrial fibrillation is known to be present in almost half of patients with degenerative mitral regurgitation at 10 years. If atrial fibrillation is found to have worse impact on TMVR, they will advocate for adjunctive concurrent treatment of atrial fibrillation in patients undergoing TMVR. Dr. Arora presented preliminary results at American College of Cardiology Scientific Sessions in Orlando, Florida in March 2018. In addition to collaborating with the Duke Clinical Research Institute on this project, UNC collaborators include Cassandra Ramm, MSN, AGNP and Matthew Cavender, MD, MPH.
and Dr. Sameer Arora (research fellow with cardiology) will be working with the coordinators of Transcatheter Valve Therapy registry at Duke Clinical Research Institute to investigate the impact of atrial fibrillation on outcomes after transcatheter mitral valve repair (TMVR). TMVR has emerged as a treatment option for severe symptomatic patients with chronic severe primary mitral regurgitation who are not surgical candidates.” Dr. Vavalle has been treating patients with this ground-breaking treatment at UNC since initiating the structural heart disease program in 2014. Atrial fibrillation is known to be present in almost half of patients with degenerative mitral regurgitation at 10 years. If atrial fibrillation is found to have worse impact on TMVR, they will advocate for adjunctive concurrent treatment of atrial fibrillation in patients undergoing TMVR. Dr. Arora presented preliminary results at American College of Cardiology Scientific Sessions in Orlando, Florida in March 2018. In addition to collaborating with the Duke Clinical Research Institute on this project, UNC collaborators include Cassandra Ramm, MSN, AGNP and Matthew Cavender, MD, MPH.
Research into Outcomes of Transcatheter Aortic Valve Replacement
 Led by Dr. John Vavalle, the UNC Heart Valve team has been prolific with numerous peer-reviewed publications focused on transcatheter aortic valve replacement (TAVR). This includes multiple meta-analyses focused on comparing TAVR with surgery. Their published work has been crucial in advancing the technology of TAVR which is now approved for intermediate surgical-risk patients with ongoing trials to investigate TAVR in low-surgical-risk patients. With growing recognition in the field of TAVR, the UNC heart valve team has written numerous invited editorials and review articles. They also addressed concerns about longevity of TAVR valves in an important work focusing on durability of TAVR valves published in The American Journal of Cardiology. As it becomes difficult to differentiate TAVR from surgery on the basis of mortality alone, they are now focusing on comparing the resource utilization between the two interventions. Their aim is to continue their efforts in the field of TAVR to help expand this technology to all patients with severe aortic stenosis. Collaborators on these project included Cassandra Ramm, MSN, AGNP, Thomas Caranasos, MD and Matthew Cavender, MD, MPH.
Led by Dr. John Vavalle, the UNC Heart Valve team has been prolific with numerous peer-reviewed publications focused on transcatheter aortic valve replacement (TAVR). This includes multiple meta-analyses focused on comparing TAVR with surgery. Their published work has been crucial in advancing the technology of TAVR which is now approved for intermediate surgical-risk patients with ongoing trials to investigate TAVR in low-surgical-risk patients. With growing recognition in the field of TAVR, the UNC heart valve team has written numerous invited editorials and review articles. They also addressed concerns about longevity of TAVR valves in an important work focusing on durability of TAVR valves published in The American Journal of Cardiology. As it becomes difficult to differentiate TAVR from surgery on the basis of mortality alone, they are now focusing on comparing the resource utilization between the two interventions. Their aim is to continue their efforts in the field of TAVR to help expand this technology to all patients with severe aortic stenosis. Collaborators on these project included Cassandra Ramm, MSN, AGNP, Thomas Caranasos, MD and Matthew Cavender, MD, MPH.