The Division of Gastroenterology and Hepatology, and its affiliated Center for Gastrointestinal Biology and Disease, is a national leader in gastrointestinal and liver disease research. In 2018, the Division received more than $23 million in research awards, one of the largest funding bases in UNC’s School of Medicine, including both basic and clinical departments. Remarkably, the division also performed more than 20,000 endoscopic procedures, saw more than 20,000 patients in clinic, and published over 277 papers. Members serve on the Board of Editors of Clinical Gastroenterology and Hepatology (Evan Dellon, MD, MPH) and the Editorial Advisory Board of the American Journal of Gastroenterology (Millie Long, MD, MPH). Following are highlights from the division.
Anne Peery, MD, MSCR, documents burden of digestive diseases

Anne Peery, MD, MSCR, is an assistant professor in the Division of Gastroenterology and Hepatology. She has published a series of landmark papers on diverticulosis and diverticulitis including national guidelines on the management of acute diverticulitis. Her research is supported by a K23 award from the NIH.
Dr. Peery and her colleagues recently published an updated report in Gastroenterology estimating the burden and cost for gastrointestinal diseases in the United States. Estimates were based on the most currently available administrative claims from commercial and Medicare Supplemental plans, data from the GI Quality Improvement Consortium Registry, and multiple national databases. The annual health care expenditures for gastrointestinal diseases totaled $135.9 billion. Yearly, there were more than 54.4 million ambulatory visits with a primary diagnosis for a GI disease, 3.0 million hospital admissions, and 540,500 all-cause 30-day readmissions. There were 266,600 new cases of GI cancers diagnosed and 144,300 cancer deaths. Each year, there were 97,700 deaths from non-malignant GI diseases. An estimated 11.0 million colonoscopies, 6.1 million upper endoscopies, 313,000 flexible sigmoidoscopies, 178,400 upper endoscopic ultrasound examinations, and 169,500 ERCP procedures were performed annually.
Because statistics quantifying the burden of gastrointestinal diseases are valuable in public health research, decision-making, priority-setting, and resource allocation, these reports are highly cited. A previous version of the paper published in 2015 has been cited 346 times (Google Scholar).
Hans Herfarth, MD, PhD, answers clinical important question about methotrexate monotherapy in patients with active ulcerative colitis
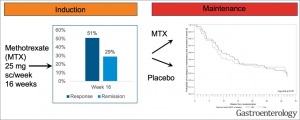
Methotrexate (MTX) was shown to be clinically effective in patients with Crohn’s disease in two landmark papers published in the 1990’s. The therapeutic efficacy in ulcerative colitis, on the other hand, has been debated for decades. To finally determine whether MTX has a clinical benefit in patients with active UC, Hans Herfarth, MD, PhD conducted an investigator-initiated, prospective, placebo controlled, multi-center 48-week-long study with the acronym MERIT-UC. The trial included a 16-week open-label methotrexate (MTX) induction period followed by a 32-week double-blind placebo-controlled maintenance period. The trial was funded by an $8.1 million grant from NIH and the results recently published in Gastroenterology (see figure).
MERIT-UC, which was the first randomized, placebo-controlled maintenance trial in patients with mild-moderate UC, finally answered the 30-year-old question of whether MTX provides a long-term clinical benefit in patients with UC. Whereas MTX had a limited efficacy to induce steroid-free response or remission in combination with a standardized steroid taper in the open label induction period, MTX did not show better efficacy as a maintenance treatment than placebo in preventing relapse in patients with UC.
Donna Evon, PhD, studies behavioral and psychological aspects of chronic liver disease
Donna Evon, PhD, Associate Professor of Medicine, is a behavioral researcher and clinical psychologist whose research interests include behavioral and psychosocial aspects of chronic liver disease, patient experiences with medical therapies and intervention development.
In 2015, Dr. Evon and colleagues received a 3-year, $2.5 million grant from The Patient-Centered Outcomes Research Institute (PCORI) to characterize patient experiences during and after direct acting antiviral (DAA) therapy for chronic hepatitis C viral (HCV) infection. New DAA therapies have been FDA approved since 2014, but real-world, patient-reported experiences taking these new therapies have been lacking.
The Patient-Reported Outcomes Project of HCV-TARGET (“PROP UP”) was a multi-center prospective observational study that enrolled 1,600 patients with HCV who were starting DAA regimens at 11 hepatology centers across the U.S. Dr. Evon’s team also included an HCV Patient Engagement Group who assisted with prioritizing the study outcomes and selecting the best patient-reported outcomes measures (PROMs). The team is investigating study outcomes such as changes in HCV symptoms and functioning during and up to one-year after DAA therapy.
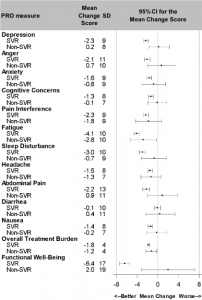
Retention in the study remained over 96% and PRO data collection rates were over 95% at all follow-up time points. Findings from the PROP UP study are now being published. At baseline, the most prevalent HCV-associated symptoms were pain, fatigue, and sleep disturbance and these symptoms were more often associated with sociodemographic and psychiatric markers, rather than liver disease markers. During DAA therapy, symptom and functioning mean scores only changed slightly. However, unlike PRO data derived from DAA registration trials which report ubiquitous positive experiences during DAA therapy, the PROP UP investigators found a heterogeneous range of experiences in this real-world, non-trial cohort; some patients experienced substantial symptom improvements while others experienced significant worsening of symptoms. The investigators also found that patients who achieved viral cure experienced clinical improvements in fatigue, sleep, and functional well-being whereas those who did not achieve cure seem to experience minimal improvements. UNC investigators collaborating on the PROP UP study include Drs. Michael Fried, Carol Golin, Paul Stewart, Jipcy Amador and Bryce Reeve (Duke).
Evan Dellon, MD, MPH, conducting clinical trials to advance care for eosinophilic esophagitis patients
Dr. Evan Dellon’s research focuses on eosinophilic esophagitis (EoE), a chronic allergy/immune-mediated esophageal disease. This condition is characterized by symptoms of esophageal dysfunction and a marked eosinophilic infiltration in the esophageal mucosa, which leads to esophageal strictures and other structural changes. Dr. Dellon has a wide research portfolio, with ongoing studies related to the epidemiology, pathogenesis, diagnosis, and treatment of eosinophilic esophagitis. The overall goal of his research is to improve the lives of patients with EoE by learning how to better diagnose, treat, and monitor the condition.
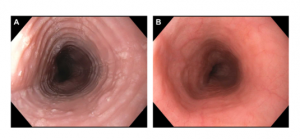
Dr. Dellon has recently conducted a comparative effectiveness treatment for the two most common types of swallowed, or “topical” steroids, which are used to treat EoE – budesonide and fluticasone. Aqueous budesonide is mixed into a viscous slurry and swallowed, whereas fluticasone is dispensed from an asthma inhaler. Instead of breathing in the medication, it is puffed into the mouth and swallowed. In this randomized, double-blind, double-dummy, clinical trial conducted at UNC and led by Dr. Dellon, 111 patients with an incident diagnosis of EoE received either a budesonide slurry plus a placebo inhaler, or fluticasone inhaler plus a placebo slurry (Dellon et al, Gastroenterology). The results showed that initial treatment of EoE with either budesonide or fluticasone resulted in a significant decrease in esophageal eosinophil counts and improved dysphagia symptoms and endoscopic features (see figure), but that budesonide was not superior to fluticasone. This has important clinical implications, and allows patients and providers to choose the medication and formulation that is best for them, based on preference for ease of administration for a given patient, convenience, and cost.
The UNC Center for Esophageal Diseases and Swallowing is a busy clinical research center, with multiple ongoing clinical trials for EoE. Dr. Dellon is currently leading an investigator-initiated multi-site randomized controlled trial of mepolizumab for treatment of EoE. This medication is a monoclonal antibody to IL-5, which is highly involved in EoE pathogenesis. This study is currently enrolling.
Shehzad Z. Sheikh, MD, PhD – Understanding the pathogenesis and finding a cure for Crohn’s Disease
Shehzad Sheikh, MD, PhD, is an Associate Professor of Medicine. His work is supported by an R01 award from the NIH: Integrative genetic and genomic analyses in the inflammatory bowel disease. R01 DK104828. Dr. Sheikh’s clinical and research focus is understanding the pathogenesis and finding a cure for Crohn’s disease (CD). CD is a lifelong condition characterized by a fluctuating course of gastro-intestinal inflammation with repeated flares and remissions. Persistent inflammation can result in complications, such as a narrowing of the large intestine (strictures) or perforations in the intestinal wall, both of which often require surgical treatment and severely compromise the quality of life of CD patients. Because disease course widely varies among patients and there is no “one size fits all” treatment, determining which patients are at high risk for poor clinical outcome is a critical problem in the management of Crohn’s disease.
Dr. Sheikh’s group recently conducted a study in adult and pediatric patients with Crohn’s disease published October 4, 2018 in the Journal of Clinical Investigation (JCI) Insight. They found that a set of biomolecules known as microRNAs, specifically microRNA-31 (miR-31), can help predict which patients with Crohn’s disease are at higher risk for the development of severe problems that may require surgical removal of the large intestine. These analyses found that in adults, low colonic miR-31 expression at the time of surgery was associated with worse disease outcomes, requiring an end ileostomy and later recurrence of disease. In children, low colonic miR-31 expression at the time of diagnosis was found to be associated with future development of strictures that required surgery.
For such a clinically heterogenous disease, this kind of molecular phenotyping is a major step towards personalization of medical therapy. These results add to a series of papers from his group combining genomic technologies with rigorous validation in patient-derived, disease-relevant cell systems to develop molecular markers with prognostic utility.
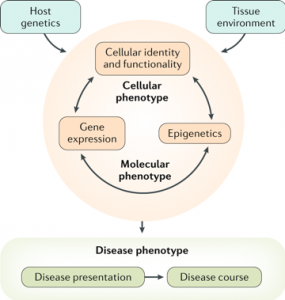
Dr. Sheikh and his colleagues provide their conceptual approach to studying CD (see figure) in the context of recent advances in the field in a recent review paper “Redefining the IBDs using genome-scale molecular phenotyping” published in Nat Rev Gastroenterol Hepatology in February 20, 2019. In recent years there has been great success in deriving molecular signatures that classify several cancers into subtypes, including studies performed here at UNC. These subtypes, then, have been shown to be useful in determining cancer progression and response to specific therapies. Dr. Sheikh’s collaborative group is extending this work to uncover molecular subtypes of Crohn’s disease to not only increase our understanding of the root causes of the disease and the vast clinical heterogeneity, but also to more strategically use current therapies and provide the basis for new therapies that specifically target these subtypes.
A major advancement in Dr. Sheikh’s recent work has been the use of formalin fixed paraffin embedded tissue samples routinely used in the clinic to preserve patient tissue samples. Dr. Sheikh’s group was accurately able to detect microRNA expression that was not degraded in fixed samples opening up future studies to a wealth of patient samples from across the country to further validate microRNA-31 and a suite of other microRNAs as prognostic markers of Crohn’s disease.
Dr. Sheikh’s research group is highly collaborative and funded through various foundations (Crohn’s and Colitis Foundation of America, American Gastroenterological Association, Broad Medical Research Program, Helmsley Charitable Trust) and the NIH (NIDDK and NIEHS). Results of work from his lab have resulted in integration of ‘multi-omics’ data including genome wide sequencing studies of chromatin, RNA, small RNA and microbial 16S rRNA with IBD disease phenotype.