Although mutated versions of the protein Cdh1 have not been found in cancers, the protein’s degradation at a key moment during the cell cycle may spur on cancerous cell division.

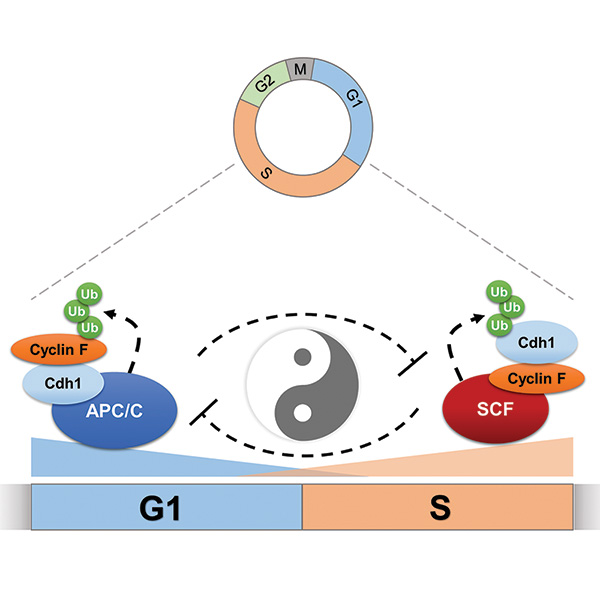
CHAPEL HILL, NC – How a cell transitions from the G1 phase of the cell cycle to the S phase – a fundamental process of cell division for all eukaryotic cells on Earth – has been a long-studied question in biology. Now UNC School of Medicine scientists discovered that this boundary is regulated by a pair of large enzyme complexes that attack each other in turn to form a molecular switch, which eventually allows the cell cycle to enter into S phase.
The discovery, published in today in Cell Reports, raises the possibility that future drugs could target this enzyme interaction to help stop the uncontrolled division of cancerous cells. Above all, though, it illuminates the workings of one of the most important processes in biology.
“Our work describes a key piece of the puzzle for how this fundamental process works,” said Michael J. Emanuele, PhD, study senior author, assistant professor in department of pharmacology, and member of the UNC Lineberger Comprehensive Cancer Center. “We did not focus on suppressing cancer cell division in this study, but given what we’ve found we think it’s definitely worth investigating further.”
Read the full article by Mark Derewicz on the SOM News
Abstract
The anaphase promoting complex/cyclosome (APC/ C) is an ubiquitin ligase and core component of the cell-cycle oscillator. During G1 phase, APC/C binds to its substrate receptor Cdh1 and APC/CCdh1 plays an important role in restricting S-phase entry and maintaining genome integrity. We describe a recip- rocal feedback circuit between APC/C and a second ubiquitin ligase, the SCF (Skp1-Cul1-F box). We show that cyclin F, a cell-cycle-regulated substrate receptor (F-box protein) for the SCF, is targeted for degradation by APC/C. Furthermore, we establish that Cdh1 is itself a substrate of SCFcyclin F. Cyclin F loss impairs Cdh1 degradation and delays S-phase entry, and this delay is reversed by simultaneous removal of Cdh1. These data indicate that the coordi- nated, temporal ordering of cyclin F and Cdh1 degra- dation, organized in a double-negative feedback loop, represents a fundamental aspect of cell-cycle control. This mutual antagonism could be a feature of other oscillating systems.
Read the journal article: Choudhury et al., APC/C and SCFcyclin F Constitute a Reciprocal Feedback Circuit Controlling S-Phase Entry, Cell Reports, 16 (12):3359–3372, 2016: http://dx.doi.org/10.1016/j.celrep.2016.08.058
The other co-authors of the study were first author Rajarshi Choudhury, PhD, senior post-doctoral fellow and research associate, Thomas Bonacci, PhD, postdoctoral fellow; and graduate students Anthony Arceci, Christine A. Mills, and Jennifer L. Kernan, all of the Emanuele Laboratory at UNC. Other authors included and James A. DeCaprio, MD, and graduate student Timothy B. Branigan, both of the Dana Farber Cancer Center and Harvard Medical School; and Daniel J. Burke, PhD, and Debojyoti Lahiri, PhD, a postdoc in Burke’s lab at NC State University.
