The Hahn, Kuhlman and Kasai labs developed a genetically encoded probe that can label and optically erase synapses that were enlarged or generated by motor learning. Dense labeling of synapses was found in a small subset of pyramidal neurons, and irradiation with blue light erased motor memory.
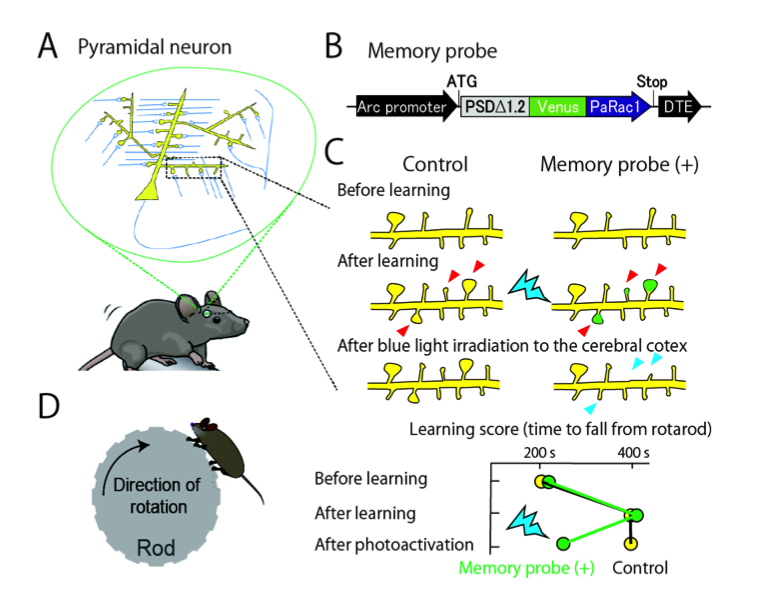
In the cerebral cortex, billions of neurons are connected by synapses to form elaborate neuronal networks. Notably, most excitatory synapses are formed on spiny protrusions of neurons known as dendritic spines (Fig. A). These spines are dynamically modifiable, and their structures correlate with synaptic connectivity.
Neuronal spines were hypothesized to be memory elements, but this could not be directly tested as there was no means to alter the large number of spines involved in memory formation. Collaborative research between The University of Tokyo and the University of North Carolina led to development of an optical probe which selectively labels enlarged or newly generated spines and, in addition, can shrink and eliminate the labeled spines by blue laser irradiation of living brains (Fig. B,C). The genetically encoded probe was transfected into most neurons in mouse motor cortex. After mice were trained to improve motor task performance, irradiation of their motor cortex with blue light was used to eliminate learning-induced spine changes, resulting in reversal of the improvements to task performance (Fig. D). This indicated that spine changes are necessary for memory formation.
We also found that the memory spines were distributed in a small fraction (10-20%) of neurons, within which many spines (about 100) were labeled, suggesting that a specific strongly connected circuit is responsible for specific memory. This “synaptic optogenetics” will be a powerful tool for further clarifying brain functions and disorders.
Links to abstract and/or article
PubMed: http://www.ncbi.nlm.nih.gov/pubmed/26352471
Nature News & Views: Lu, J. & Zuo, Yi (2015) Forgetfulness illuminated. Nature 525, 324-325.
Faculty of 1000: http://f1000.com/prime/725776471
Reference
Hayashi-Takagi, Yagishita, Nakamura, Shirai, Wu, Loshbaugh, Kuhlman, Hahn, and Kasai (2015). Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333-338. DOI: 10.1038/nature15257
