The thesis work of Dr. Tatyana Bodrug, an alumna of the Brown Lab, on polyubiquitination by APC/C is published in Nature Structural & Molecular Biology!
A collaborative team of scientists across the globe, led by the Nicholas Brown lab with Dr. Tatyana Bodrug as the first author and including Kaeli A. Welsh, Derek L. Bolhuis, and Ethan Paulаkonis, in collaboration with the David Haselbach lab at IMP Vienna, Ellen Zhong lab at Princeton University, Wei Zhang lab at the University of Guelph, and Klaus Hahn lab at UNC Pharmacology have employed time-resolved cryogenic electron microscopy (cryo-EM) to investigate the workings of a crucial protein complex involved in substrate polyubiquitination.
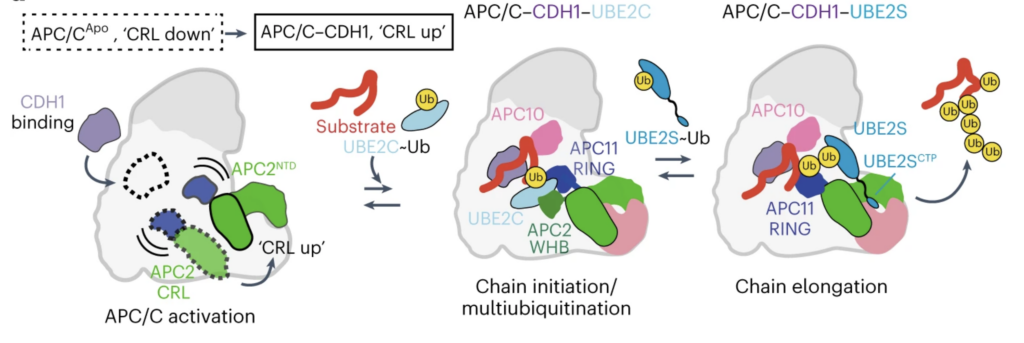
Anaphase-promoting complex/cyclosome (APC/C) is a ubiquitin ligase driving the cell cycle. Working alongside co-enzymes, APC/C recognizes important substrates and orchestrates targeted polyubiquitination to degrade these unwanted proteins within the cell. The specific mechanisms of APC/C in this process remained an unsolving puzzle. To unravel this complexity, the research team employed time-resolved cryogenic electron microscopy (cryo-EM) to unveil the dynamic conformational changes occurring within the human APC/C during polyubiquitination. The interaction between APC/C and its substrate is reconstructed using Deep Reconstructing Generative Networks (cryoDRGN). Together, their work showcased how the modification of substrates drives polyubiquitination and sheds light on how a ubiquitin ligase assembles a proteasomal degradation signal. These findings represent a significant leap forward in our understanding of cellular regulation and protein degradation, with future implications for the development of targeted therapies for various diseases.