The CFTR Functional Analysis Core
Director: Martina Gentzsch, Ph.D.
Multiple research strategies for treatment of cystic fibrosis (CF) are currently being explored. Translating CF therapeutic strategies from basic research to clinical studies requires the assessment of drug candidates in physiologically relevant assays. To support translational CF research, the CFTR Functional Analysis Core pursues four objectives:
 |
|---|
|
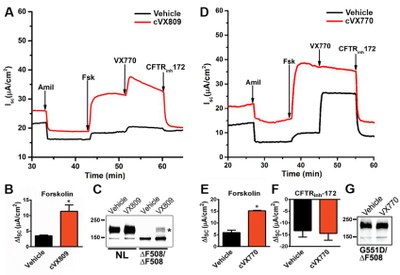
Ussing Chamber Systems for measuring electrophysiological characteristics of tissue samples and cell cultures. |
1) Analyze ion channel properties and correction efficiency of human bronchial epithelial (HBE) cells from harvested CF lungs to provide a full characterization report to investigators.
2) Measure ion transport function of CFTR and ENaC in HBE and human nasal epithelial (HNE) cultures by bioelectric and organoid assays to asses efficacy of candidate therapies.
3) Evaluate CFTR expression and processing by biochemical analyses to assess efficacy of CFTR modulation strategies.
4) Validate the suitability of reagents, supplies, and techniques for optimizing HBE and HNE cell integrity.
Although numerous investigators using services provided by the CFTR Functional Analysis Core focus on restoration of CFTR function in CF cells by small-molecule pharmacological interventions, our services offer broad applications to multiple research programs that examine gene targeting/therapy, modifier genes, and impact of environmental factors such as inflammation, infection, and smoking on epithelial ion transport. Furthermore, our services allow for the correlation of CFTR activity with other outcome measures such as airway surface liquid hydration and mucus clearance.
Core Personnel:
Acknowledgement:
Contact Information
Phone:(919) 966-7058
Fax:(919)966-5178
E-mail: gentzsch@med.unc.edu