The Animal Models Core
Director: Wanda K. O’Neal, PhD; Co-Director: Alessandra Livraghi-Butrico, PhD
Introduction
The Marsico Lung Institute/UNC Cystic Fibrosis Center Animal Models Core is dedicated to providing access and specialized expertise to conduct in vivo studies with animal models (primarily mouse) that provide insights into pulmonary disease pathophysiology and treatment. Core users are typically collaborating academic investigators and industry partners, through Spirovation. The Animal Models Core has been involved in the generation and characterization of over 25 lines of genetically modified mice (transgenic, knockout, or knockin) for a variety of collaborators over the years, and has also conducted studies in other species (rabbit, hamster, rat, guinea pig) for investigator-initiated projects. The Mouse Models Core laboratory is located on the seventh floor of Marsico Hall (Rm 7229L, 7224, 7122a), with mouse colonies housed in the Genetic Medicine Division of Comparative Medicine (DCM) facility, and dedicated DCM BSL2 cubicles in Marsico Hall (B107, 7229AA). Main activities of the Core include:
Mouse line development and colony management:
The development of new lines is highly dependent upon a collaboration with the UNC Transgenic Mouse Core, and more recently to the use of transgenic lines obtained from international resources, such as KOMP, EUCOMM, and the MRRCC. Once founders are identified, lines are validated, expanded and maintained to generate experimental cohorts or provide breeder pairs to external investigators. Publications utilizing these mouse models in a variety of context can be found here.
Phenotyping:
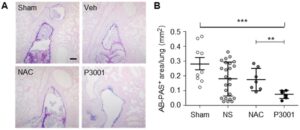
The Animal Models Core supports research covering many aspects of lung disease biology, including airway mucus clearance, bacterial and viral pathogenesis, injury/repair, and inflammation. Expertise in several aspects of in vivo experimentation is provided including model selection, experimental challenges, pharmacological treatments, and comprehensive respiratory phenotyping for both upper and lower airways. Routine techniques include: survival and non-survival surgeries, health status monitoring, bronchoalveolar lavage, middle ear lavage, tissue harvest, preparation of samples for cytological, histological, microbiological, biochemical, immunological, and rheological analyses, and primary culture of tracheal and nasopharyngeal airway cells. Histological services (processing, embedding, sectioning) are routinely provided through UNC-based Histology Cores (Pathology Services Core, Histology Research Core, or Center for Gastrointestinal Biology and Disease Histology Core).
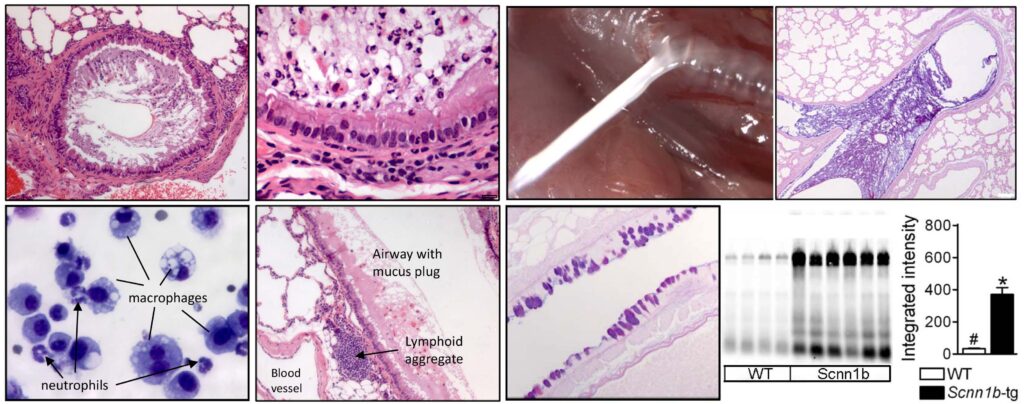
Collaboration with experts in the field also allows access to specialized equipment/techniques, including aerosol administration through a nose-only inhalation exposure system (DSI Inhalation exposure system, in collaboration with Dr. Gregory Smith); pulmonary function test (SCIREQ flexivent system), mucociliary clearance assay (in collaboration with Dr. Barbara Grubb), nasal mucociliary clearance by pinhole gamma scintigraphy, in collaboration with the Center for Environmental Medicine, Asthma and Lung Biology); characterization of ex vivo tissue or cell culture bioelectrical properties (Drs. Barbara Grubb and Martina Gentzsch).

Regulatory compliance:
The Animal Models Core provides guidance and assistance in matters regarding regulatory compliance with UNC Environmental Health and Safety (EHS), Office of Animal Care and Use (OACU), and Division of Comparative Medicine (DCM) guidelines.
Spirovation:
Spirovation is a specialty contract research organization (CRO) affiliated with the Marsico Lung Institute and its mission is to empower breakthrough drug development in respiratory medicine by providing leading scientific expertise and the broadest array of translational medicine capabilities in respiratory research, including those provided by the Animal Models core.
Personnel

Associate Professor
woneal@med.unc.edu
Director

Associate Professor
Alessandra_livraghi@med.unc.edu
Co-Director

Research Specialist

Research Specialist

Research Specialist

Research Specialist

Research Specialist
Allison joined the Animal Models Core in January of 2010. Allison graduated from Texas A&M University where she obtained a Bachelor’s of Science in Biomedical Science. Before moving to Chapel Hill, she worked at the Baylor College of Medicine and The University of Texas- Medical Branch for Dr. Paul Overbeek in Molecular Biology and Dr. Neal Waxham in Neurobiology, respectively. She is our chief mouse colony manager and actively participates in phenotyping efforts.
Tyler Gray, BS
Research Specialist
Tyler joined the Animal Models Core in July of 2022. Tyler graduated from Christopher Newport University where he obtained a Bachelor of Science in Cellular and Molecular Biology in 2022. Before moving to Chapel Hill, he worked at the Department of Neuroscience Research at Christopher Newport University for Dr. Matthew Campolattaro. Tyler contributes to experimental efforts of the Animal Models Core including NIH contracts for establishment of chronic bacterial infections.
Joey Kennedy, MS
Research Specialist
Joey joined the Animal Models Core in March of 2023. He graduated from the University of Mississippi in 2019, where he obtained a Master of Science in Biological Sciences. Before moving to Chapel Hill, Joey worked as a Research Associate and then Manager of the Animal Metabolism and Behavior Core at Pennington Biomedical Research Center in Baton Rouge, LA. Joey is active in various research projects involving experimental challenges and phenotyping in the Animal Models Core.
Jazmin Snead, MAS
Research Specialist
Jazmin has worked in Dr. Livraghi-Butrico’s lab and the MLI Animal Models Core since January of 2023. Jazmin graduated from North Carolina State University, where she obtained a Master of Animal Science in 2023. Before joining the Livraghi-Butrico lab, she worked as a Research Support Technician for the Division of Comparative Medicine at UNC. Jazmin specializes in laboratory animals handling, western blots, immunohistochemistry, and gastrointestinal and pulmonary research.
Tsion Tegicho, BS
Research Specialist
Tsion Tegicho began working with the Animal Models Core in August 2023. She graduated from Carthage College where she obtained a Bachelor of Science in Neuroscience in 2023. Tsion works with the Animal Models Core and Dr. Matthew Wolfgang’s lab, contributing to experimental efforts to establish chronic bacterial infections in mouse models.
Acknowledgement:
Funded by the NIH/NIDDK (P30 DK065988) and the CFF (BOUCHE19R0).
Contact Information
125 Mason Farm RoadThe University of North Carolina at Chapel Hill
Campus Box #7248
Chapel Hill, NC 27599
Phone: (919) 843-1097
Fax: (919) 966-5178 Email: woneal@med.unc.edu
Email: alessandra_livraghi@med.unc.edu
Core Publications:
- Livraghi-Butrico A, Franklin TB, Wolfgang MC. The rat takes the cheese: a novel model of CFTR-dependent chronic bacterial airway infection. Editorial. Eur Resp J. 2022 Sep 7;60(3):2200832. doi: 10.1183/13993003.00832-2022.
- Biering SB, Sarnik SA, Wang E, Zengel JR, Leist SR, Schäfer A, Sathyan V, Hawkins P, Okuda K, Tau C, Jangid AR, Duffy CV, Wei J, Gilmore RC, Alfajaro MM, Strine MS, Nguyenla X, Van Dis E, Catamura C, Yamashiro LH, Belk JA, Begeman A, Stark JC, Shon DJ, Fox DM, Ezzatpour S, Huang E, Olegario N, Rustagi A, Volmer AS, Livraghi-Butrico A, Wehri E, Behringer RR, Cheon DJ, Schaletzky J, Aguilar HC, Puschnik AS, Button B, Pinsky BA, Blish CA, Baric RS, O’Neal WK, Bertozzi CR, Wilen CB, Boucher RC, Carette JE, Stanley SA, Harris E, Konermann S, Hsu PD. Genome-wide bidirectional CRISPR screens identify mucins as host factors modulating SARS-CoV-2 infection. Nat Genet. 2022 Aug;54(8):1078-1089. doi: 10.1038/s41588-022-01131-x. PMID: 35879412. PMCID: PMC9355872.
- Kim N, Kwak G, Rodriguez J, Livraghi-Butrico A, Zuo X, Simon V, Han E, Shenoy SK, Pandey N, Mazur M, Birket SE, Kim A, Rowe SM, Boucher R, Hanes J, Suk JS. Inhaled gene therapy of preclinical muco-obstructive lung diseases by nanoparticles capable of breaching the airway mucus barrier. Thorax. 2022 Aug;77(8):812-820. doi: 10.1136/thoraxjnl-2020-215185. PMID: 34697091; PMCID: PMC9129924.
- Grubb BR, Livraghi-Butrico A. Animal models of cystic fibrosis in the era of highly effective modulator therapies. Review. Curr Opin Pharmacol. 2022 Jun;64:102235. doi: 10.1016/j.coph.2022.102235. PMID: 35576754; PMCID: PMC9386876.
- Rogers TD, Button B, Kelada SNP, Ostrowski LE, Livraghi-Butrico A, Gutay MI, Esther CR Jr, Grubb BR. Regional Differences in Mucociliary Clearance in the Upper and Lower Airways. Front Physiol. 2022 Mar 9;13:842592. doi: 10.3389/fphys.2022.842592. PMID: 35356083; PMCID: PMC8959816.
- Xu J, Livraghi-Butrico A, Hou X, Rajagopalan C, Zhang J, Song J, Jiang H, Wei HG, Wang H, Bouhamdan M, Ruan J, Yang D, Qiu Y, Youming X, Barrett RP, McClellan SA, Mou H, Wu Q, Chen X, Rogers TD, Wilkinson KJ, Gilmore RC, Esther CR Jr, Zaman K, Liang X, Sobolic M, Hazlett L, Zhang K, Frizzell RA, Gentzsch M, O’Neal WK, Grubb BR, Chen YE, Boucher RC, Sun F. Phenotypes of CF rabbits generated by CRISPR/Cas9-mediated disruption of the CFTR gene. JCI Insight. 2021 Jan 11;6(1):e139813. doi: 10.1172/jci.insight.139813. PMID: 33232302. PMCID: PMC7821608.
- Sigmon JS, Blanchard MW, Baric RS, Bell TA, Brennan J, Brockmann GA, Burks AW, Calabrese JM, Caron KM, Cheney RE, Ciavatta D, Conlon F, Darr DB, Faber J, Franklin C, Gershon TR, Gralinski L, Gu B, Gaines CH, Hagan RS, Heimsath EG, Heise MT, Hock P, Ideraabdullah F, Jennette JC, Kafri T, Kashfeen A, Kulis M, Kumar V, Linnertz C, Livraghi-Butrico A, Lloyd KCK, Lutz C, Lynch RM, Magnuson T, Matsushima GK, McMullan R, Miller DR, Mohlke KL, Moy SS, Murphy CEY, Najarian M, O’Brien L, Palmer AA, Philpot BD, Randell SH, Reinholdt L, Ren Y, Rockwood S, Rogala AR, Saraswatula A, Sassetti CM, Schisler JC, Schoenrock SA, Shaw GD, Shorter JR, Smith CM, St Pierre CL, Tarantino LM, Threadgill DW, Valdar W, Vilen BJ, Wardwell K, Whitmire JK, Williams L, Zylka MJ, Ferris MT, McMillan L, Manuel de Villena FP. Content and Performance of the MiniMUGA Genotyping Array: A New Tool To Improve Rigor and Reproducibility in Mouse Research. Genetics. 2020 Dec;216(4):905-930. doi: 10.1534/genetics.120.303596. Epub 2020 Oct 16. PMID: 33067325; PMCID: PMC7768238.
- Brinks V, Lipinska K, de Jager M, Beumer W, Button B, Livraghi-Butrico A, Henig N, Matthee B. The Cystic Fibrosis-Like Airway Surface Layer Is not a Significant Barrier for Delivery of Eluforsen to Airway Epithelial Cells. J Aerosol Med Pulm Drug Deliv. 2019 Oct;32(5):303-316. doi: 10.1089/jamp.2018.1502. Epub 2019 May 22. PMID: 31120356; PMCID: PMC6781260.
- Yin W, Livraghi-Butrico A, Sears PR, Rogers TD, Burns KA, Grubb BR, Ostrowski LE. Mice with a Deletion of Rsph1 Exhibit a Low Level of Mucociliary Clearance and Develop a Primary Ciliary Dyskinesia Phenotype. Am J Respir Cell Mol Biol. 2019 Sep;61(3):312-321. doi: 10.1165/rcmb.2017-0387OC. PMID: 30896965; PMCID: PMC6839924.
- Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, Martino MB, Dang H, Abzhanova A, Lin JM, Hull-Ryde EA, Volmer AS, Randell SH, Livraghi-Butrico A, Deng Y, Scherer PE, Stripp BR, O’Neal WK, Boucher RC. XBP1S regulates MUC5B in a promoter variant-dependent pathway in IPF airway epithelia. Am J Respir Crit Care Med. 2019 Jul 15;200(2):220-234. doi: 10.1164/rccm.201810-1972OC. PMID: 30973754. PMCID: PMC6635783.
- Ehre C, Rushton ZL, Wang B, Hothem LN, Morrison CB, Fontana NC, Markovetz MR, Delion MF, Kato T, Villalon D, Thelin WR, Esther CR Jr, Hill DB, Grubb BR, Livraghi-Butrico A, Donaldson SH, Boucher RC. An Improved Inhaled Mucolytic to Treat Airway Muco-Obstructive Diseases. Am J Respir Crit Care Med. 2019 Jan 15;199(2):171-180. doi: 10.1164/rccm.201802-0245OC. PMID: 30212240. PMCID: PMC6353008.
- Rogers TD, Ostrowski LE, Livraghi-Butrico A, Button B, Grubb BR. Mucociliary Clearance in Mice Measured by Tracking Trans-tracheal Fluorescence of Nasally Aerosolized Beads. Sci Rep. 2018 Oct 3;8(1):14744. doi: 10.1038/s41598-018-33053-2. PMID: 30282981. PMCID: PMC6170422.
- Chen G, Volmer AS, Wilkinson KJ, Deng Y, Jones LC, Yu D, Bustamante-Marin XM, Burns KA, Grubb BR, O’Neal WK, Livraghi-Butrico A, Boucher RC. Role of Spdef in the regulation of Muc5b expression in the airways of naïve and muco-obstructed mice. Am J Respir Cell Mol Biol. 2018 Sep;59(3):383-396. doi: 10.1165/rcmb.2017-0127OC. PMID: 29579396. PMCID: PMC6189647.
- Saini Y, Lewis B, Yu D, Dang H, Livraghi-Butrico A, Del Piero F, O’Neal W, Boucher R. Effect of LysM+ macrophage depletion on lung pathology in mice with chronic bronchitis. Physiol Rep. 2018 Apr;6(8):e13677. doi: 10.14814/phy2.13677. PMID: 29667749. PMCID: PMC5904692.
- Duncan GA, Kim N, Colon-Cortes Y, Rodriguez J, Mazur M, Birket SE, Rowe SM, West NE, Livraghi-Butrico A, Boucher RC, Hanes J, Aslanidi G, Suk JS. An Adeno-Associated Viral Vector Capable of Penetrating the Mucus Barrier to Inhaled Gene Therapy. Mol Ther Methods Clin Dev. 2018 Mar 22;9:296-304. doi: 10.1016/j.omtm.2018.03.006. PMID: 30038933; PMCID: PMC6054694.
- Livraghi-Butrico A, Wilkinson K, Volmer A, Gilmore R, Rogers T, Caldwell R, Burns K, Esther C Jr., Mall M, Boucher R, O’Neal W, Grubb B. Lung disease phenotypes caused by over-expression of combinations of alpha, beta, and gamma subunits of the epithelial sodium channel in mouse airways. Am J Physiol Lung Cell Mol Physiol. 2018 Feb 1;314(2):L318-L331. doi: 10.1152/ajplung.00382.2017. PMID: 29074490. PMCID: PMC5866504.
- Rowson-Hodel AR, Wald JH, Hatakeyama J, O’Neal WK, Stonebraker JR, VanderVorst K, Saldana MJ, Borowsky AD, Sweeney C, Carraway KL 3rd. Membrane Mucin Muc4 promotes blood cell association with tumor cells and mediates efficient metastasis in a mouse model of breast cancer. Oncogene. 2018 Jan 11;37(2):197-207. doi: 10.1038/onc.2017.327. PMID: 28892049. PMCID: PMC5930013.
- Donoghue LJ*, Livraghi-Butrico A*, McFadden KM, Thomas JM, Chen G, Grubb BR, O’Neal WK, Boucher RC, Kelada SNP. Identification of trans protein QTL for secreted airway mucins in mice and a causal role for Bpifb1. Genetics. 2017 Oct;207(2):801-812. doi: 10.1534/genetics.117.300211. PMID: 28851744. PMCID: PMC5629340.
- Livraghi-Butrico A, Grubb BR, Wilkinson K, Volmer AS, Burns KA, Evans C, O’Neal WK, Boucher RC. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2017 Mar;10(2):395-407. doi: 10.1038/mi.2016.63. PMID: 27435107. PMCID: PMC5250616.
- Sesma JI, Weitzer CD, Livraghi-Butrico A, Dang H, Donaldson S, Alexis NE, Jacobson KA, Harden TK, Lazarowski ER. UDP-glucose promotes neutrophil recruitment in the lung. Purinergic Signal. 2016 Dec;12(4):627-635. doi: 10.1007/s11302-016-9524-5. Epub 2016 Jul 15. PMID: 27421735; PMCID: PMC5124001.
- Grubb BR, Livraghi-Butrico A, Rogers TD, Yin W, Button B, Ostrowski LE. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am J Physiol Lung Cell Mol Physiol. 2016 May 1;310(9):L860-7. doi: 10.1152/ajplung.00015.2016. PMID: 26968767. PMCID: PMC4867354.
- Saini Y, Wilkinson KJ, Terrell KA, Burns KA, Livraghi-Butrico A, Doerschuk CM, O’Neal WK, Boucher RC. Neonatal pulmonary macrophage depletion coupled to defective mucus clearance increases susceptibility to pneumonia and alters pulmonary immune responses. Am J Respir Cell Mol Biol. 2016 Feb;54(2):210-21. doi: 10.1165/rcmb.2014-0111OC. PMID: 26121027; PMCID: PMC4821038.
- Yu D, Davis RM, Aita M, Burns KA, Clapp PW, Gilmore RC, Chua M, O’Neal WK, Schlegel R, Randell SH, C Boucher R. Characterization of Rat Meibomian Gland Ion and Fluid Transport. Invest Ophthalmol Vis Sci. 2016 Apr 1;57(4):2328-43. doi: 10.1167/iovs.15-17945. PMID: 27127933; PMCID: PMC4855829.
- Saini Y, Dang H, Livraghi-Butrico A, Kelly EJ, Jones LC, O’Neal WK, Boucher RC. Gene expression in whole lung and pulmonary macrophages reflects the dynamic pathology associated with airway surface dehydration. BMC Genomics. 2014 Sep 10;15(1):726. doi: 10.1186/1471-2164-15-726. PMID: 25204199; PMCID: PMC4247008.
- Sesma JI, Kreda SM, Okada SF, van Heusden C, Moussa L, Jones LC, O’Neal WK, Togawa N, Hiasa M, Moriyama Y, Lazarowski ER. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol. 2013 May 15;304(10):C976-84. doi: 10.1152/ajpcell.00371.2012. Erratum in: Am J Physiol Cell Physiol. 2014 Feb 15;306(4):C415. PMID: 23467297; PMCID: PMC3651637.
- Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O’Neal WK, Grubb BR. Loss of Cftr function exacerbates the phenotype of Na(+) hyperabsorption in murine airways. Am J Physiol Lung Cell Mol Physiol. 2013 Apr 1;304(7):L469-80. doi: 10.1152/ajplung.00150.2012. PMID: 23377346; PMCID: PMC3627939.
- Roy M, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JA, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defense. Nature. 2013;505:412-416. doi: 10.1038/nature12807. PMID: 24317696; PMCID: PMC4001806.
- Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O’Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol. 2012 Jul;5(4):397-408. doi: 10.1038/mi.2012.17. PMID: 22419116; PMCID: PMC3377774.
- Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O’Neal WK, Sallenave JM, Pickles RJ, Boucher RC. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):16528-33. doi: 10.1073/pnas.1206552109. Erratum in: Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):5753. PMID: 23012413; PMCID: PMC3478656.
- Livraghi-Butrico A, Grubb BR, Kelly EJ, Wilkinson KJ, Yang H, Geiser M, Randell SH, Boucher RC, O’Neal WK. Genetically determined heterogeneity of lung disease in a mouse model of airway mucus obstruction. Physiol Genomics. 2012 Apr 15;44(8):470-84. doi: 10.1152/physiolgenomics.00185.2011. PMID: 22395316; PMCID: PMC3339860.
- Jones LC, Moussa L, Fulcher ML, Zhu Y, Hudson EJ, O’Neal WK, Randell SH, Lazarowski ER, Boucher RC, Kreda SM. VAMP8 is a vesicle SNARE that regulates mucin secretion in airway goblet cells. J Physiol. 2012 Feb 1;590(3):545-62. doi: 10.1113/jphysiol.2011.222091. PMID: 22144578; PMCID: PMC3379700.
- Grubb BR, O’Neal WK, Ostrowski LE, Kreda SM, Button B, Boucher RC. Transgenic hCFTR expression fails to correct β-ENaC mouse lung disease. Am J Physiol Lung Cell Mol Physiol. 2012 Jan 15;302(2):L238-47. doi: 10.1152/ajplung.00083.2011. PMID: 22003093; PMCID: PMC3349361.
- Nguyen Y, Procario MC, Ashley SL, O’Neal WK, Pickles RJ, Weinberg JB. Limited effects of Muc1 deficiency on mouse adenovirus type 1 respiratory infection. Virus Res. 2011 Sep;160(1-2):351-9. doi: 10.1016/j.virusres.2011.07.012. PMID: 21816184; PMCID: PMC3163747.
- Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011 Jul 29;286(30):26277-86. doi: 10.1074/jbc.M111.260562. PMID: 21606493; PMCID: PMC3143590.
- Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O’Neal WK, Pradervand S, Hummler E, Rossier BC, Grubb BR, Boucher RC. Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with betaENaC-overexpressing mice. J Biol Chem. 2010 Aug 27;285(35):26945-26955. doi: 10.1074/jbc.M110.151803. PMID: 20566636; PMCID: PMC2930694.
- Ostrowski LE, Yin W, Rogers TD, Busalacchi KB, Chua M, O’Neal WK, Grubb BR. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol. 2010 Jul;43(1):55-63. doi: 10.1165/rcmb.2009-0118OC. PMID: 19675306; PMCID: PMC2911571.
- Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, O’Neal WK, Boucher RC, Randell SH. Airway and lung pathology due to mucosal surface dehydration in {beta}-epithelial Na+ channel-overexpressing mice: role of TNF-{alpha} and IL-4R{alpha} signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol. 2009 Apr 1;182(7):4357-67. doi: 10.4049/jimmunol.0802557. PMID: 19299736; PMCID: PMC2659461.
- Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, Solomon GM, McQuaid DB, Noerager BD, Gaggar A, Clancy JP, O’Neal W, Sorscher EJ, Abraham E, Blalock JE. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med. 2008 Oct 15;178(8):822-31. doi: 10.1164/rccm.200712-1894OC. PMID: 18658107; PMCID: PMC2566793.
- Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, O’Neal WK, Boucher RC. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med. 2008 Apr 1;177(7):730-42. doi: 10.1164/rccm.200708-1233OC. PMID: 18079494; PMCID: PMC2277210.
- Ostrowski LE, Yin W, Diggs PS, Rogers TD, O’Neal WK, Grubb BR. Expression of CFTR from a ciliated cell-specific promoter is ineffective at correcting nasal potential difference in CF mice. Gene Ther. 2007 Oct;14(20):1492-501. doi: 10.1038/sj.gt.3302994. PMID: 17637798.
- Stonebraker JR, Wagner D, Lefensty RW, Burns K, Gendler SJ, Bergelson JM, Boucher RC, O’Neal WK, Pickles RJ. Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: Role for tethered mucins as barriers to lumenal infection. J Virol. 2004 Dec;78(24):13755-68. doi: 10.1128/JVI.78.24.13755-13768.2004. PMID: 15564484; PMCID: PMC533903.
- Okada SF, O’Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol. 2004 Nov;124(5):513-26. doi: 10.1085/jgp.200409154. PMID: 15477379; PMCID: PMC2234005.
- Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004 May;10(5):487-93. doi: 10.1038/nm1028. PMID: 15077107.
- Zariwala M*, O’Neal WK*, Noone PG, Leigh MW, Knowles MR, Ostrowski LE. Investigation of the possible role of a novel gene, DPCD, in primary ciliary dyskinesia. Am J Respir Cell Mol Biol. 2004 Apr;30(4):428-34. doi: 10.1165/rcmb.2003-0338RC. PMID: 14630615. *authors contributed equally to manuscript.
- Ostrowski LE, Hutchins JR, Zakel K, O’Neal WK. Targeting expression of a transgene to the airway surface epithelium using a ciliated cell-specific promoter. Mol Ther. 2003 Oct;8(4):637-45. doi: 10.1016/s1525-0016(03)00221-1. PMID: 14529837.