The MLI Tissue Procurement and Cell Culture Core
Director: Scott H. Randell, PhD, Professor of Cell Biology and Physiology and Professor in Medicine
Laboratory Manager: Leslie Fulcher
How to Order Cells and Custom Media
Our Mission
Well-differentiated human airway epithelial cell cultures are instrumental for studying basic and applied aspects of cell biology and physiology relevant to cystic fibrosis (CF) and its treatment. The Marsico Lung Institute Tissue Procurement and Cell Culture Core (MLI TPC) was established to provide standardized cell cultures for CF research. The MLI TPC serves as a central source of normal, CF and disease control cells, tissues and fluids for a wide array of uses. We currently offer seven different cell types and three different types of growth media, which are all available for purchase via iLabs. The MLI TPC has prepared cells from approximately 8,150 human tissue specimens over the past 36 years, producing over 210 x 109 viable primary and passage one human airway epithelial cells between 2015 to 2019.
Current Functions
1) Tissue Procurement
Obtain nasal tissues and tracheo-bronchiolar segments from normal, CF, and disease control humans as sources of airway epithelial cells (hAE). The MLI TPC then utilizes the harvested hAE cells for culture, for ex vivo experiments, for RNA and proteins representative of in vivo conditions, and for in situ hybridization and immunohistochemistry. As a service to the greater CF research community (Function 6), the MLI TPC accepts tissues from other institutions through longstanding sources including local and distant outpatient surgical centers such as the UNC Lung Transplant Program, Carolina Donor Services, International Institute for the Advancement of Medicine (IIAM, Edison, NJ), and the National Disease Research Interchange (NDRI, Philadelphia, PA). The MLI TPC also accepts tissues from highly motivated donors nationally and internationally. Ongoing sources of CF tissues from independently recruited lung transplant programs include Loyola University, Maywood IL, Vanderbilt University, Medical College of Wisconsin, Israel, and Duke University Medical Center. Furthermore, in collaboration with the Cystic Fibrosis Foundation Therapeutics (CFFT) and NDRI, explanted CF lungs from multiple lung transplant centers are sent to UNC for processing.
2) Airway Epithelial Cell Isolation and Culture
a) Isolate epithelial cells from excised human nasal tissues, including endosinus mucosa, and cartilaginous bronchi of human lungs for distribution to investigators.
b) Prepare and maintain primary epithelial cell cultures, such as primary alveolar epithelial cells, microvascular endothelial cells and/or fibroblasts from the tissues noted above. The Core provides support for the preparation of well-differentiated airway epithelial cultures from passaged, or cryopreserved and thawed human cells, on permeable substrates.
c) Optimize and standardize epithelial culture conditions to replicate the gene expression, morphological differentiation, and physiologic functions of airways.
The MLI TPC also provides passage 1 airway epithelial cells, like primary cells, they too are suitable for producing well-differentiated cultures on porous supports. Representative sections of well-differentiated nasal and bronchial airway epithelial cell cultures are shown in Figure 1.
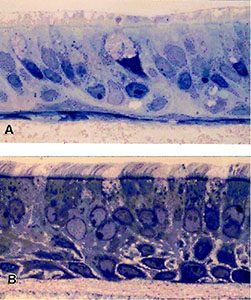
Routine MLI TPC procedures have significantly improved our ability to study differentiation-dependent functions (Figure 2) and have also increased the number and area of well-differentiated cultures produced from each sample. Possessing the ability to store primary cells at differing stages of maturity from the same patient sample allows repeat experiments with the same specimen. Additionally, we can perform simultaneous experiments with replicate cultures derived from multiple patients.
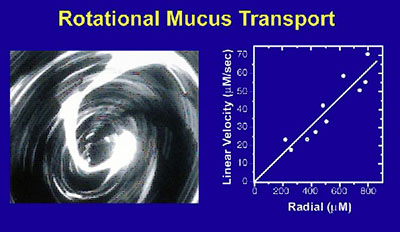
The in-house production of BEGM and ALI media has also resulted in significant cost savings and a deeper understanding of cell culture among staff. We are focused on producing cultures that accurately mirror in vivo gene expression, morphology, and physiology and which reproduce known differences between CF and non-CF individuals in vivo. Supplying large numbers of cultures, and large batches of media, in response to changing investigator needs is one of the major MLI TPC functions.
3) Collect Airway Surface Liquid (ASL) from in vivo and in vitro Samples
Critical testing of current advances in CF pathogenesis requires direct measurement of the chemical composition of human ASL. We have consistently collected and distributed in vivo airway mucus secretions for the study of biochemical composition, biophysical properties and as the source of the supernatant of mucopurulent material (SMM) from CF lungs. Additionally, ASL from well-differentiated cultures is used to stimulate air-liquid interface (ALI) cultures and serves as the basis for key experiments related to novel concepts in CF. The MLI TPC provides luminal contents of CF lungs explanted during transplant or removed at autopsy and harvests ASL from in vitro sources.
4) Genetic Manipulation of Cell Cultures
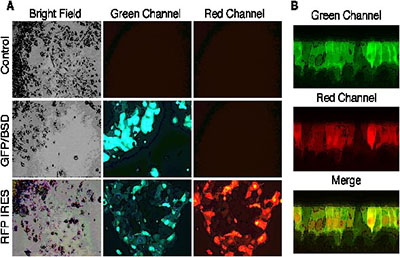
Use adenovirus, adeno-associated virus, lentivirus (see Figure 3) and retrovirus vectors in combination with chemical treatment as necessary, to assist in the production of genetically manipulated, well-differentiated primary airway epithelial cell cultures.
5) Create and Characterize Novel Cell Lines
Use recent advances in cell immortalization technology to create new cell lines and characterize their biochemical properties, morphology and electrophysiologic function. The goal of the TPC is to create new and better immortalized cells retaining a greater capacity to recapitulate normal airway epithelial function. We have used retroviral transduction to serially introduce SV40 ER or HPV E6/E7 and hTERT into non-CF and CF cells. Their growth potential, morphology, biochemical features and electrophysiologic properties are studied using typical methods. Our ongoing observations have revealed that cells immortalized by SV40 ER or HPV E6/E7 and hTERT, when grown at an air-liquid-interface, are not optimal for CF-related investigation. This has led to novel studies using Bmi-1 oncogene to create superior cell lines (AJP 2009). Six novel cell lines using the Bmi-1 oncogene (3 normal and 3 deltaF508 homozygous CF) have been characterized (see Figure 4).
6) Translation of Technology and Reagents to the Greater CF Research Community
Our Core strongly observes the philosophy of developing non-proprietary technology and supporting its widespread translation. By providing material and consultative support, the MLI TPC allows others to develop or improve upon their own in-house cell culture capabilities. This involves the shipment of cells or media, hosting visitors for training, and publication of methods specifically related to improvements in human airway cell culture. In addition to hosting periodic training sessions for visitors from the US, England, Ireland, Switzerland, Canada, France, and Israel for the purpose of technology transfer, the MLI TPC consults with distant users as they encounter experimental difficulties related to their cell model work. The MLI TPC’s standing as a recharge center has allowed for a more accessible and efficient user experience. We are able to provide immortal cell lines at minimal expense, custom cell line creation for those who have sent us tissue specimens using CRC technology, and offer customized media based on manager approval.
Some of our latest updated methods have been published as a part of the Methods in Molecular Biology (MIMB) series, with the title “Epithelial Cell Culture Protocols.” The MLI TPC strives to meet the needs of academic, UNC, and commercial interests, when possible. Consultative support including advice, protocols, and more can be found on this site. If there are any requests outside of the information provided, feel free to contact the Laboratory Manager with the link provided at the top of the page.
7) New Initiatives Based on Investigator Needs

The TPC is responsive to the changing needs of UNC MLI investigators and provides material support for phasic motion and cyclic compressive stress studies, chambers for exposure to hypoxic conditions, mouse airway epithelial cell cultures, human lung vascular endothelial cell cultures and human alveolar epithelial cell cultures. The TPC has applied new methods enabling routine provision of well-differentiated tracheal epithelial cell cultures from normal mice as well as cultures from genetically manipulated mice, including CF mice, mice over-expressing the epithlial Na+ channel, Coxsackie adenovirus receptor expressing mice, caveolin knockout mice and other genetically unique strains.
- Laboratory: The laboratories are fully equipped for general laboratory tasks including tissue culture, immunostaining, in-situ hybridization, cell transfection, Western blotting, manipulation of DNA and RNA including gene cloning, and Southern and Northern blotting.
- Dr. Randell has approved recombinant DNA protocols and BSL2 level laboratories acceptable for adeno-, retro- and lenti-viral infection of primary cells.
- Office Equipment: Supplied with 13 state-of-the-art personal desktop computers with appropriate software for data analysis, data acquisition, and image analysis.
- Three printers (two of which are color), a fax machine, and a copier/scanner are available.
- Offices: Dr. Randell (Room 1117) and the MLI TPC manager (Room 1119) have offices in Marsico Hall, as well as carrels for up to 13 laboratory members.
- Equipped with 7 laminar flow biological safety cabinets, 3 dual-chamber CO2 tissue culture incubators, benchtop centrifuges and inverted and dissecting microscopes.
- The Core utilizes a cold room, autoclaves, 18megOhm distilled water supply (DRS 1 High Purity Loop deionized water polisher), -80 and –20 freezers, and 7 liquid nitrogen cell storage tanks.
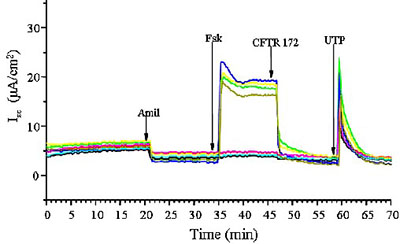
- An eight-station Ussing chamber apparatus and a newly installed two plate TECC 24 electro-physiology station are available (Figure 4).
- The CF Center, and our interdepartmental relationships, grants the Core access to linear immunoblotting capabilities for protein analysis, neon nucleofection system, multiple real-time PCR machines and more.

Tissue Culture Core’s Anaerobic Chamb
Core Protocols
- BMI Instructions
- Cell Passaging with Accutase
- Cell Count
- Cell Passaging Double Trypsinization
- Coating Plates
- Freeze and Thaw Protocol
- Coating Inserts — HPC-Collagen IV
- Cell Plating Guidelines
- Begm and ALI media
- BEAS Cells- Media
- SPOC media
- CFT1 Culture Media
- HBE1 Culture Media
- Maintenance of HBE Cells on Plastic
- Primary HBE Instructions
- Well-Differentiated Cell Cultures
Information and Links
Novel human bronchial epithelial cell lines for cystic fibrosis research
Well-Differentiated Human Airway Epithelial Cell Culture
Primary Epithelial Cell Models for Cystic Fibrosis Research
Human Bronchial Epithelial Cells and Custom Media – Order Here
Tissue Core Personnel

Scott H. Randell, PhD
Director

Leslie Fulcher
Laboratory Manager

Allison Williams
Research Specialist

Husein Syed
Student Assistant
Outings

Christmas 2020!






Contact Information
1119 Marsico HallCampus Box #7248
The University of North Carolina at Chapel Hill
Chapel Hill, NC 27599
(919) 966-7055
Fax:(919) 966-5178


