The Mucus/Mucin Biochemistry and Biophysics Core
Director: Brian Button, PhD; Co-Investigators: Camille Ehre, PhD, David B. Hill, PhD, and Mehmet Kesimer, PhD
Introduction
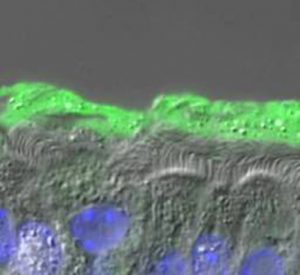
The mucus clearance system of the lung represents a key innate defense system that protects the airway surface against constant exposure to inhaled infectious and noxious particles. Abnormal clearance of mucus is an important contributor to the phenotype of patients with chronic bronchitis (CB) due to: 1) environmental causes such as cigarette smoke in the chronic bronchitic component of COPD; 2) genetic causes, such as cystic fibrosis (CF); or 3) “allergic” diseases, such as in asthma. So, why, given their complex origin, does the mucus transport fail in these diseases? Perhaps the best-known phenotype that correlates with reduction of clearance in CF, COPD, and asthma is the increased production and accumulation of thick, “sticky” mucus in the airways. The result of reduced clearance is mucus-plugged airways and persistent bacterial infections.
In health, mucus is composed of roughly 98% water and 2% solids materials, consisting of mucins (~0.5%), which represent the main “gel-forming” polymers secreted by glands and superficial goblet cells, globular proteins (~0.5%), salt (~0.9%). In CF, however, dysregulation of ion transport (CFTR) leads to unregulated fluid absorption, mucin hyperconcentration (>6% solids), and collapse of the airway surface layers (see Fig. 1). Our understanding of how a small change in mucus concentration leads to such a catastrophic failure of mucus clearance has been recently greatly advanced by our “gel-on-brush” model of the airway surface. This model posits two gels on the airway surface, i.e., a secreted gel mucus layer and a tethered mucin periciliary layer, which better fits the physiologic and biophysical properties of mucus transport. This new model emphasizes the role of the relative mucin concentrations of the mucus layer and the periciliary layer that predictably mediate mucus transport (flow) and the cessation of mucus transport.
Given the importance of understanding the role that alterations in the mucus layer plays in CF pathogenesis, the UNC Mucus Biochemistry and Biophysics Core’s goal is to provide Core users unique data describing the biochemical and biophysical properties of the mucus layer and its interaction with the cell surface. This core is broken down into three distinct functional divisions. In the first Core component (Mucus/mucin biochemistry), we will provide a service to quantify the concentration of given mucus samples for both total mucus concentration (measured as % solids) and the absolute relative contributions of each secreted mucin (e.g. MUC5AC and MUC5B) in a Core user-supplied sample (sputum, BAL, excised mucus, etc.). Our Core will also provide quantitation of extracellular DNA, which has been shown be elevated in lung diseases such as CF and PCD as a result of necrotic death of inflammatory and epithelial cells. The release of DNA has been suggested form a filamentous network that is difficult to clear by cilia- and cough-mediated clearance, making it an important target for therapeutic agents designed to improve clearance.
In the second Core component (Mucus biophysics), we will provide Core users information regarding the biophysical properties of the mucus layer and it’s interaction with the cell surface, both critical for understanding the “clearability” of the mucus layer. Here, we will provide measurements of mucus “osmotic pressure”, a key determinant of the water drawing properties of the mucus and the interaction of the mucus and PCL. We also propose to provide macroscopic and microscopic rheological characterization of mucus, a measure of the “flowability” the mucus. Increases in mucus viscoelastic properties, as measured by classical rheological techniques, are the best-characterized properties of mucus dehydration. Studies will also focus on quantifying the “cohesive strength” of the mucus, which will provide valuable information to Core users regarding how difficult it is to “tear apart” mucus, key for maintaining cough clearance.
Based on our novel model of how the mucus and the PCL layers are structured and interact, we have shown that mucus dehydration produces “sticky” mucus that adheres to epithelial cells, which worsen as a function of disease severity, such as during acute exacerbations. Because of the importance for understanding how the mucus interacts with the cell surface, assays performed in this Core are designed to directly allow investigators to quantify the adhesive interaction of the mucus with the cell surface and assess how this interaction is altered by therapeutics.
Finally, the third Core component (Mucus Clearance), focuses on understanding how changes in the biochemical and biophysical properties of the mucus combine to regulate/alter the rate of mucus clearance. Here, we will utilize a series of novel in vitro techniques to provide Core user’s data quantifying both cilia- and cough-mediated clearance and assess the impact of mucus alterations by disease state, e.g. mucus concentration, or the effect of Core users therapeutic agents
Core Faculty Members:




Acknowledgement:
Contact Information
Brian Button, Ph.D.6017 Thurston-Bowles Bldg.
Campus Box #7248
The University of North Carolina at Chapel Hill
Chapel Hill, NC 27599
Phone: (919) 966-5823
Fax:(919) 966-5178