The Cystic Fibrosis Molecular/Functional Measurement Core
Director: Martina Gentzsch, Ph.D.
Co-Director: Wanda O’Neal, Ph.D.
 |
|---|
|
The Cystic Fibrosis Molecular/Functional Measurement Core is designed to provide services to analyze the efficacy of treatments that target different stages in the pathogenic sequelae of CF disease development.
|
To support urgently required translational Cystic Fibrosis (CF) research, the Cystic Fibrosis Molecular/Functional Measurement Core of the University of North Carolina CF Research and Translation Core Center is pursuing the following objectives:
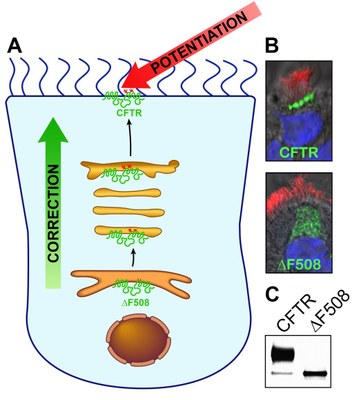
- Evaluation of pre-clinical drug candidates in vitro in primary human airway and intestinal planar epithelial CF cultures by measurement of ion transport, CFTR maturation and surface expression, drug pharmacokinetics, and airway surface liquid properties.
- Evaluation of pre-clinical drug candidates ex vivo in organoid models (i.e. colonospheres, nasospheres, and bronchospheres) and various epithelial tissues (mouse distal colon, jejunum, gallbladder, trachea, bronchi, nasopharnyx, and human rectal biopsies). Services provided are quantitative organoid swelling assays, functional assessment of ion transport in tissues, and biochemical assessment of CFTR rescue in organoids and tissues.
- Evaluation of pre-clinical drug candidates in vivo in relevant mouse models of CF and other lung diseases using a variety of assays to determine the efficacy of therapeutic treatments on ion transport defects, CFTR processing, defective mucus clearance, chronic infection, and inflammation. Services provided in this objective include mouse maintenance and distribution, pharmacological treatments, pharmacokinetic analyses, and phenotyping including collection of bronchoalveolar lavage, measurements of soluble mediators, airway mucus burden and clearance, nasal potential difference, salivary secretion rates, histopathology, morphometry, microbiology, and technologically advanced airway imaging.
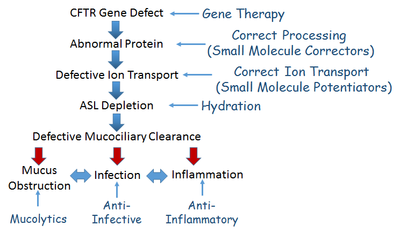
While certain assays and models provided by the Core focus on restoration of CFTR function in CF, most of our services have broad applications to multiple research programs seeking therapeutic benefit for CF. These include approaches that utilize small-molecule pharmacological interventions, gene therapy, correction of airway surface liquid hydration and mucus clearance defects, normalization of ion transport, and inflammation/infection control.
The availability of models and assays by the Cystic Fibrosis Molecular/Functional Measurement Core will provide a translational bridge that will support the rapid transfer of emerging drug candidates from the research laboratory to therapies for CF patients.
Cystic Fibrosis Molecular/Functional Measurement Core key investigators:
Martina Gentzsch, Ph.D.
Wanda O’Neal, Ph.D.
Alessandra Livraghi-Butrico, Ph.D.
Barbara Grubb, Ph.D.
Charles Esther, M.D., Ph.D.
Michael Chua, Ph.D.
Acknowledgement:
Funded by the NIH/NIDDK (P30 DK065988)
Contact Information
Martina Gentzsch, Ph.D.
Associate Professor, Department of Cell Biology and Physiology
Director, Cystic Fibrosis Molecular/Functional Measurement Core
Director, CFTR Functional Analysis Core
Marsico Lung Institute/Cystic Fibrosis Research Center
The University of North Carolina
1121 Marsico Hall CB 7248
125 Mason Farm Road
Chapel Hill, NC 27599
Phone: (919) 966-7058
Fax: (919) 966-5178
E-mail: gentzsch@med.unc.edu