***Noteworthy: A figure from a review article written by Dr. Ribeiro and Dr. Emily Hull-Ryde for Current Opinion in Pharmacology was chosen for the cover of the 2022 Respiratory Issue. The article can be found here. The cover for the 2022 Respiratory Issue, for which Dr. Ribeiro served with Dr. Martina Gentzsch as guest editors, can be previewed below.***

Specialty Areas:
Inflammation Mechanisms in CF, COPD, and Asthma
Chronology:
PhD: Duke University, 1992; Postdoctoral: NIH/NIEHS, 1993-1998; Research Associate, University of North Carolina, 1998-2001; Assistant Professor of Medicine, University of North Carolina, 2001-2010; Associate Professor of Medicine, University of North Carolina, 2010-present; Joint Associate Professor of Cell Biology and Physiology, University of North Carolina, July 2011-May 2021; Professor of Cell Biology and Physiology, University of North Carolina, May 2021-present.
Research Focus:
Research in the Ribeiro laboratory focuses on studying mechanisms of airway inflammatory responses relevant to the pathogenesis of airway diseases characterized by mucus obstruction, inflammation, and oxidative stress, such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD) and asthma. In particular, we study the functional roles of the endoplasmic reticulum (ER) and the mitochondria in the regulation of intracellular calcium (Ca2+i) signals and Ca2+i-mediated inflammation, and ER stress responses pertinent to the pathophysiology of these pulmonary diseases.
Techniques employed in the Ribeiro lab include:
- Primary culturing of human and murine airway epithelia; culturing of a variety of immortalized cell lines including airway epithelial cell lines.
- Bronchoalveolar lavages; isolation and culturing of murine and human alveolar macrophages.
- DNA, RNA and protein extraction from cells and tissues; DNA, RNA and protein quantitation; DNA cloning; RNA purification; RNase Protection Assays; PCR and RT-PCR; RNA microarrays.
- Northern and Southern blotting.
- Agarose and polyacrylamide gel electrophoresis; Western blotting.
- ELISA; immunocytochemistry; immunofluorescence; confocal microscopy.
- Measurements of intracellular calcium signals (including ER and mitochondrial calcium mobilization) by microfluorimetry and confocal microscopy.
- Assessment of intracellular reactive oxygen species.
- Assays to evaluate airway mucin production and secretion.
I joined the UNC Cystic Fibrosis Center in 1998 with 1) a strong background in calcium signaling, acquired during my postdoctoral training at the National Institute of Environmental and Health Sciences (NIEHS/NIH), and 2) a solid understanding of renal epithelial biology and membrane transport from my studies at Baylor College of Medicine and during graduate school at Duke University. Hence, the research in my lab has combined these areas of expertise to create a new field aimed at addressing fundamental questions in human airway epithelial biology involving Ca2+i-dependent responses and their role in airway inflammation.
Our studies have revealed that Ca2+i responses to infectious/inflammatory stimuli are increased in CF epithelia due to an expansion of the endoplasmic reticulum (ER) Ca2+i stores. We have subsequently shown that the ER/Ca2+i store expansion contributes to airway hyperinflammation by amplifying Ca2+i-dependent inflammatory responses and increasing the ER cpaacity for the epithelial synthesis of inflammatory mediators. The initial findings in CF epithelia have been extended to other diseased epithelia, since we have also found that ER/Ca2+i stores are also expanded in inflamed, native primary ciliary diskynesia and COPD human airway epithelia.
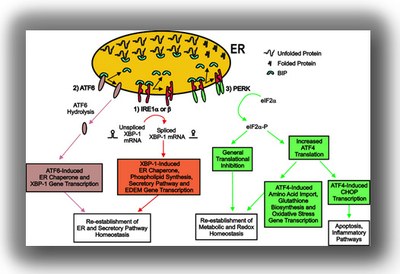
Because the ER/Ca2+i store expansion is a hallmark of several pulmonary diseases, we reasoned that it is an adaptive epithelial response that plays a key functional role in the pathophysiology of airway inflammation. We have addressed the mechanism responsible for the ER/Ca2+i store expansion during airway inflammation and found that it is mediated by activation of an ER stress response known as the unfolded protein response (UPR). In mammalian cells, activation of the UPR by increased levels of unfolded proteins in the lumen of the ER is sensed by 3 ER stress transducers, ATF6, IRE1 (a and b) and PERK. Activation of these UPR pathways results in downstream activation of signaling pathways relevant to the pathophysiology of airways diseases. (Fig. 1)
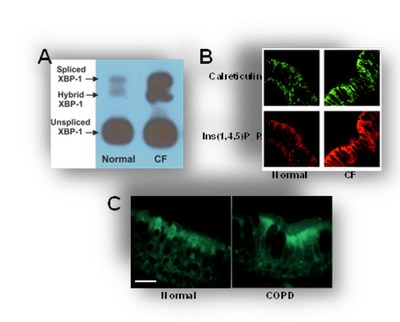
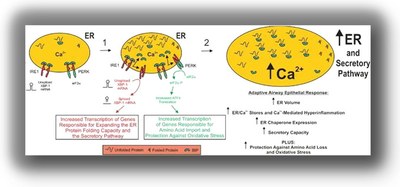
The UPR pathway responsible for the ER/Ca2+i stores is mediated by IRE1a-dependent mRNA splicing (activation) of the transcription factor X-box binding protein 1 (XBP-1). Indeed, native CF human airway epithelia exhibit up-regulation of IRE1a-dependent XBP-1 mRNA splicing coupled with up-regulation of ER/Ca2+i stores (Fig 2). Up-regulation of ER/Ca2+i stores is also a feature of native COPD human airway epithelia (Fig 2). Further studies in our lab have also implicated airway epithelia inflammation with the activation of an additional UPR pathway mediated by activating transcription factor 4 (ATF4). Activation of ATF4 is relevant to inflammatory responses, since ATF4 confers protection against oxidative stress, induces amino acid transport, and improves cellular metabolism and survival. Our current model for the roles of XBP-1 and ATF4 in airway epithelial inflammatory responses in shown in Figure 3.
The alterations in ER signaling resulting from activation of the UPR can have additional consequences for the cell biology of inflamed airway epithelia. For example, we have also reported that mitochondria are in close proximity to ER/Ca2+i stores and buffer ER/Ca2+i signals triggered by mucosal inflammatory mediators in human airway epithelia. Because mitochondrial respiration is a major source of intracellular reactive oxygen species (ROS), and mitochondrial ER/Ca2+i uptake stimulates mitochondrial respiration-dependent ROS production, we have addressed whether a direct correlation between the magnitude of ER/Ca2+i signals and the mitochondrial generation of ROS exists in inflamed airway epithelia. Our current findings suggest that the increased ER/Ca2+i signals resulting from ER/Ca2+i store expansion couple to a larger ER/Ca2+i mediated mitochondrial generation of ROS in inflamed airway epithelia. These alterations are relevant to oxidative stress responses of inflamed CF airways.
An important aspect of our research has been the development of a new model of CF airway epithelial inflammation, consisting of exposing normal airway epithelia to supernatant from mucopurulent material (SMM) from human CF airways. This model has been initially used to test the anti-inflammatory action of the macrolide antibiotic azithromycin, and has been subsequently used to test additional macrolides for anti-inflammatory effects. Please see below the additional studies we have initiated utilizing the SMM model.
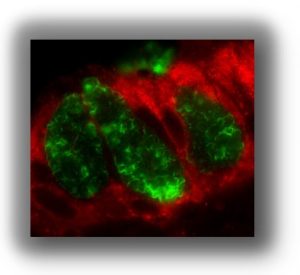
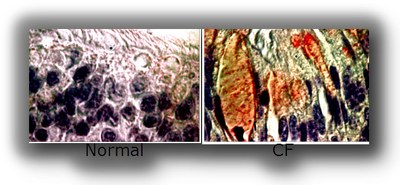
A key feature of our research involving UPR activation and airway inflammation deals with a hallmark of chronic inflammation in CF, COPD and asthmatic airways, e.g., the overproduction of mucins (Fig 4). We have published in Mucosal Immunology that the IRE1 isoform, IRE1B, is specifically expressed in mucous cells (Fig. 5) and is required for airway mucin production. These findings offer the proof of concept that IRE1B is a novel therapeutic target for the mucus overproduction characteristic of CF, COPD and asthmatic airways.
Our latest published research has uncovered a key role for the IRE1a/XBP-1 pathway in mediating hyper-inflammatory responses of human CF alveolar macrophages.
We are currently investigating the functional role of additional branches of the UPR in other aspects of pulmonary inflammation, and in cigarette smoke-induced alterations in airway epithelial function. Our long-term goal is to establish the functional importance of UPR activation in airway inflammation by performing translational studies relevant to human airway diseases, including CF, COPD and asthma. These studies will likely lead to new therapies for these pulmonary diseases, based on targeting UPR pathways.
Selected Bibliography:
- Gentzsch M, Esther CR Jr, Ribeiro CMP. Airway inflammation enhances the effectiveness of elexacaftor-tezacaftor-ivacaftor therapy for cystic fibrosis and CFTRN1303K mutation. Lancet Respir Med. 2024 Nov;12(11):e67. doi: 10.1016/S2213-2600(24)00305-9. PMID: 39395437.
- Asakura T, Okuda K, Chen G, Dang H, Kato T, Mikami Y, Schworer SA, Gilmore RC, Radicioni G, Hawkins P, Barbosa Cardenas SM, Saito M, Cawley AM, De la Cruz G, Chua M, Alexis NE, Masugi Y, Noone PG, Ribeiro CMP, Kesimer M, Olivier KN, Hasegawa N, Randell SH, O’Neal WK, Boucher RC. Proximal and Distal Bronchioles Contribute to the Pathogenesis of Non-Cystic Fibrosis Bronchiectasis (NCFB). Am J Respir Crit Care Med.2024 Feb 15;209(4):374-389. doi: 10.1164/rccm.202306-1093OC. PMID: 38016030. PMCID: PMC10878387.
- Gentzsch M, Baker B, Cholon DM, Kam CW, McKinzie CJ, Despotes KA, Boyles SE, Quinney NL, Esther CR Jr, Ribeiro CMP. Cystic fibrosis airway inflammation enables elexacaftor/tezacaftor/ivacaftor-mediated rescue of N1303K CFTR mutation. ERJ Open Res. 2024 Jan 15;10(1):00746-2023. doi: 10.1183/23120541.00746-2023. PMID: 38226069; PMCID: PMC10789252.
- Cholon DM, Greenwald MA, Higgs MG, Quinney NL, Boyles SE, Meinig SL, Minges JT, Chaubal A, Tarran R, Ribeiro CMP, Wolfgang MC, Gentzsch M. A Novel Co-Culture Model Reveals Enhanced CFTR Rescue in Primary Cystic Fibrosis Airway Epithelial Cultures with Persistent Pseudomonas aeruginosa Infection. Cells. 2023 Nov 13;12(22):2618. doi: 10.3390/cells12222618. PMID: 37998353; PMCID: PMC10670530.
- Ribeiro CMP, Higgs MG, Muhlebach MS, Wolfgang MC, Borgatti M, Lampronti I, Cabrini G. Revisiting Host-Pathogen Interactions in Cystic Fibrosis Lungs in the Era of CFTR Modulators. Int J Mol Sci. 2023 Mar 5;24(5):5010. doi: 10.3390/ijms24055010. PMID: 36902441; PMCID: PMC10003689.
- Ribeiro CMP, Gentzsch M. Editorial overview – 2022 respiratory issue: Cystic fibrosis pathophysiology, models, and novel therapies. Curr Opin Pharmacol. 2022 Dec;67:102289. doi: 10.1016/j.coph.2022.102289. PMID: 36152600.
- Figueira MF, Ribeiro CMP, Button B. Mucus-targeting therapies of defective mucus clearance for cystic fibrosis: A short review. Curr Opin Pharmacol. 2022 Aug;65:102248. doi: 10.1016/j.coph.2022.102248. PMID: 35689870. PMCID: PMC9891491.
- Ribeiro CMP, Hull-Ryde EA. Functional role of the ER stress transducer IRE1α in CF airway epithelial inflammation. Curr Opin Pharmacol. 2022 Aug;65:102258. doi: 10.1016/j.coph.2022.102258. PMID: 35749907.
- Davis ES, Ghosh A, Coakley RD, Wrennall JA, Lubamba BA, Rowell TR, Dang H, Pawlak EA, Li Q, Alexis NE, Ribeiro CMP, Tarran R. Chronic E-Cigarette Exposure Alters Human Alveolar Macrophage Morphology and Gene Expression. Nicotine Tob Res. 2022 Feb 14;24(3):395-399. doi: 10.1093/ntr/ntab186. PMID: 34519792; PMCID: PMC8842413.
- Ribeiro CMP, Gentzsch M. Impact of Airway Inflammation on the Efficacy of CFTR Modulators. Cells. 2021 Nov 22;10(11):3260. doi: 10.3390/cells10113260. PMID: 34831482; PMCID: PMC8619863.
- Ribeiro CMP, McElvaney NG, Cabrini G. Editorial: Novel Anti-Inflammatory Approaches for Cystic Fibrosis Lung Disease: Identification of Molecular Targets and Design of Innovative Therapies. Front Pharmacol. 2021 Nov 4;12:794854. doi: 10.3389/fphar.2021.794854. PMID: 34867428; PMCID: PMC8632627.
- Gentzsch M, Cholon DM, Quinney NL, Martino MEB, Minges JT, Boyles SE, Guhr Lee TN, Esther CR Jr, Ribeiro CMP. Airway Epithelial Inflammation In Vitro Augments the Rescue of Mutant CFTR by Current CFTR Modulator Therapies. Front Pharmacol. 2021 Mar 30;12:628722. doi: 10.3389/fphar.2021.628722. PMID: 33859562; PMCID: PMC8042279.
- Hull-Ryde EA, Minges JT, Martino MEB, Kato T, Norris-Drouin JL, Ribeiro CMP. IRE1α Is a Therapeutic Target for Cystic Fibrosis Airway Inflammation. Int J Mol Sci. 2021 Mar 17;22(6):3063. doi: 10.3390/ijms22063063. PMID: 33802742; PMCID: PMC8002512.
- Rimessi A, Pozzato C, Carparelli L, Rossi A, Ranucci S, De Fino I, Cigana C, Talarico A, Wieckowski MR, Ribeiro CMP, Trapella C, Rossi G, Cabrini G, Bragonzi A, Pinton P. Pharmacological modulation of mitochondrial calcium uniporter controls lung inflammation in cystic fibrosis. Sci Adv. 2020 May 6;6(19):eaax9093. doi: 10.1126/sciadv.aax9093. PMID: 32494695; PMCID: PMC7202873.
- Amatngalim GD, Ribeiro CMP. Getting neural about airway gland secretion. Eur Respir J. 2020 Apr 16;55(4):2000466. doi: 10.1183/13993003.00466-2020. PMID: 32300022.
- O’Neal WK, Ribeiro CMP. “Shocking” the System to Achieve Efficient Gene Targeting in Primary Human Airway Epithelia. Am J Respir Cell Mol Biol. 2020 Mar;62(3):279-280. doi: 10.1165/rcmb.2019-0360ED. PMID: 31633992; PMCID: PMC7055690.
- Murphy SV, Ribeiro CMP. Cystic Fibrosis Inflammation: Hyperinflammatory, Hypoinflammatory, or Both? Am J Respir Cell Mol Biol. 2019 Sep;61(3):273-274. doi: 10.1165/rcmb.2019-0107ED. PMID: 30951377; PMCID: PMC6839932.
- Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, Martino MB, Dang H, Abzhanova A, Lin JM, Hull-Ryde EA, Volmer AS, Randell SH, Livraghi-Butrico A, Deng Y, Scherer PE, Stripp BR, O’Neal WK, Boucher RC. XBP1S Regulates MUC5B in a Promoter Variant-Dependent Pathway in Idiopathic Pulmonary Fibrosis Airway Epithelia. Am J Respir Crit Care Med. 2019 Jul 15;200(2):220-234. doi: 10.1164/rccm.201810-1972OC. Erratum in: Am J Respir Crit Care Med. 2019 Oct 15;200(8):1074. PMID: 30973754; PMCID: PMC6635783.
- Gentzsch M, Cholon DM, Quinney NL, Boyles SE, Martino MEB, Ribeiro CMP. The cystic fibrosis airway milieu enhances rescue of F508del in a pre-clinical model. Eur Respir J. 2018 Dec 20;52(6):1801133. doi: 10.1183/13993003.01133-2018. PMID: 30287473; PMCID: PMC6482470.
- Webster MJ, Reidel B, Tan CD, Ghosh A, Alexis NE, Donaldson SH, Kesimer M, Ribeiro CMP, Tarran R. SPLUNC1 degradation by the cystic fibrosis mucosal environment drives airway surface liquid dehydration. Eur Respir J. 2018 Oct 4;52(4):1800668. doi: 10.1183/13993003.00668-2018. PMID: 30190268; PMCID: PMC6547379.
- Rimessi A, Bezzerri V, Salvatori F, Tamanini A, Nigro F, Dechecchi MC, Santangelo A, Prandini P, Munari S, Provezza L, Garreau de Loubresse N, Muller J, Ribeiro CMP, Lippi G, Gambari R, Pinton P, Cabrini G. PLCB3 Loss of Function Reduces Pseudomonas aeruginosa-Dependent IL-8 Release in Cystic Fibrosis. Am J Respir Cell Mol Biol. 2018 Oct;59(4):428-436. doi: 10.1165/rcmb.2017-0267OC. PMID: 29668297.
- Abdullah LH, Coakley R, Webster MJ, Zhu Y, Tarran R, Radicioni G, Kesimer M, Boucher RC, Davis CW, Ribeiro CMP. Mucin Production and Hydration Responses to Mucopurulent Materials in Normal versus Cystic Fibrosis Airway Epithelia. Am J Respir Crit Care Med. 2018 Feb 15;197(4):481-491. doi: 10.1164/rccm.201706-1139OC. PMID: 29099608; PMCID: PMC5821906.
- Ribeiro CM, Lubamba BA. Role of IRE1α/XBP-1 in Cystic Fibrosis Airway Inflammation. Int J Mol Sci. 2017 Jan 9;18(1):118. doi: 10.3390/ijms18010118. PMID: 28075361; PMCID: PMC5297752.
- Lubamba BA, Jones LC, O’Neal WK, Boucher RC, Ribeiro CM. X-Box-Binding Protein 1 and Innate Immune Responses of Human Cystic Fibrosis Alveolar Macrophages. Am J Respir Crit Care Med. 2015 Dec 15;192(12):1449-61. doi: 10.1164/rccm.201504-0657OC. PMID: 26331676; PMCID: PMC4731720.
- Zhu Y, Abdullah LH, Doyle SP, Nguyen K, Ribeiro CM, Vasquez PA, Forest MG, Lethem MI, Dickey BF, Davis CW. Baseline Goblet Cell Mucin Secretion in the Airways Exceeds Stimulated Secretion over Extended Time Periods, and Is Sensitive to Shear Stress and Intracellular Mucin Stores. PLoS One. 2015 May 29;10(5):e0127267. doi: 10.1371/journal.pone.0127267. PMID: 26024524; PMCID: PMC4449158.
- Okada SF, Ribeiro CM, Sesma JI, Seminario-Vidal L, Abdullah LH, van Heusden C, Lazarowski ER, Boucher RC. Inflammation promotes airway epithelial ATP release via calcium-dependent vesicular pathways. Am J Respir Cell Mol Biol. 2013 Nov;49(5):814-20. doi: 10.1165/rcmb.2012-0493OC. PMID: 23763446; PMCID: PMC3931099.
- Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, Ron D, O’Neal WK, Ribeiro CM. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol. 2013 May;6(3):639-54. doi: 10.1038/mi.2012.105. PMID: 23168839; PMCID: PMC4031691.
- Ribeiro CM, O’Neal WK. Endoplasmic reticulum stress in chronic obstructive lung diseases. Curr Mol Med. 2012 Aug;12(7):872-82. doi: 10.2174/156652412801318791. PMID: 22697344.
- Ribeiro CM, Boucher RC. Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc Am Thorac Soc. 2010 Nov;7(6):387-94. doi: 10.1513/pats.201001-017AW. PMID: 21030518; PMCID: PMC3136959.
- Ribeiro CM, Hurd H, Wu Y, Martino ME, Jones L, Brighton B, Boucher RC, O’Neal WK. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS One. 2009 Jun 5;4(6):e5806. doi: 10.1371/journal.pone.0005806. PMID: 19503797; PMCID: PMC2688381.
- Martino ME, Olsen JC, Fulcher NB, Wolfgang MC, O’Neal WK, Ribeiro CM. Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J Biol Chem. 2009 May 29;284(22):14904-13. doi: 10.1074/jbc.M809180200. PMID: 19321437; PMCID: PMC2685672.
- Livraghi A, Mall M, Paradiso AM, Boucher RC, Ribeiro CM. Modelling dysregulated Na+ absorption in airway epithelial cells with mucosal nystatin treatment. Am J Respir Cell Mol Biol. 2008 Apr;38(4):423-34. doi: 10.1165/rcmb.2007-0177OC. PMID: 17989361; PMCID: PMC2274946.
- Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O’neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005 May 6;280(18):17798-806. doi: 10.1074/jbc.M410618200. PMID: 15746099.
- Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia. J Biol Chem. 2005 Mar 18;280(11):10202-9. doi: 10.1074/jbc.M410617200. PMID: 15647273.
